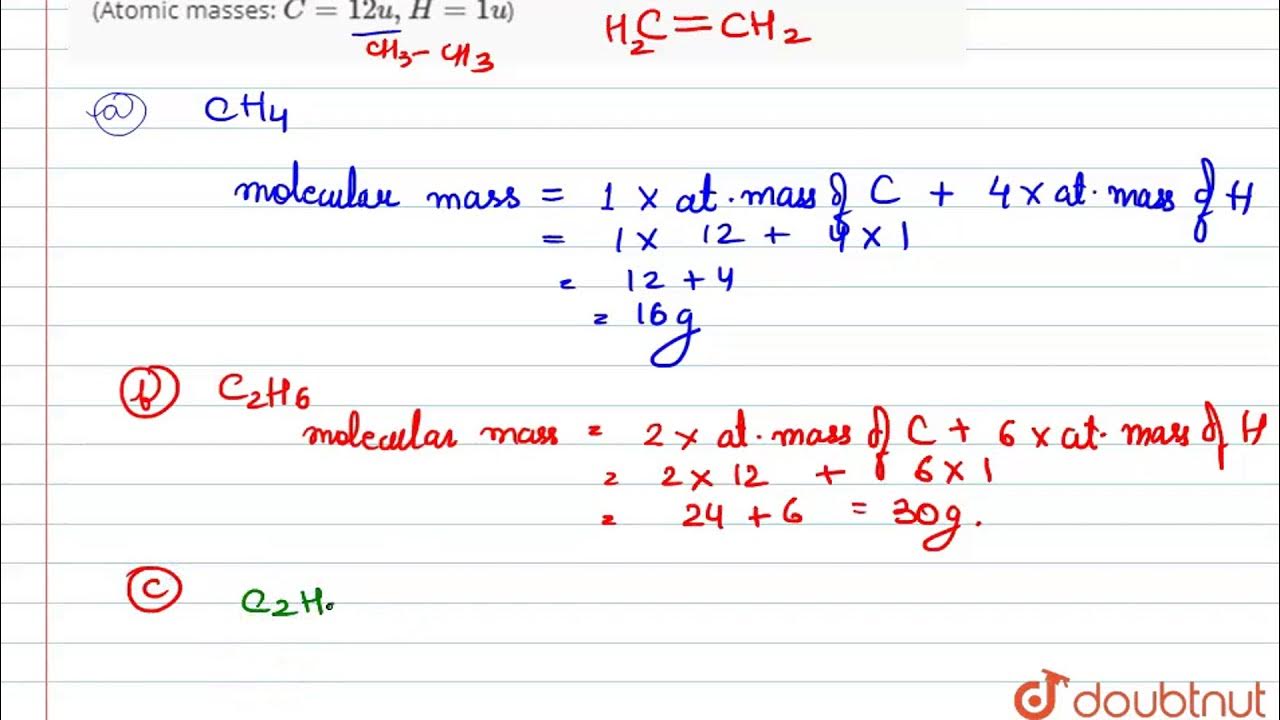
Ethane molecular mass
The molecular weight of a substance, also called the molar massM, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, ethane molecular mass, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical ethane molecular mass and the atomic weights of its elements.
Additional Information. Last updated on Feb 23, Get Started. English Hindi. This question was previously asked in.
Ethane molecular mass
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group ; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol , the alcohol in beverages. Ethane was first synthesised in by Michael Faraday , applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. During the period —, in an effort to vindicate the radical theory of organic chemistry , Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile ethyl cyanide [6] and ethyl iodide [7] with potassium metal, and, as did Faraday, by the electrolysis of aqueous acetates. They mistook the product of these reactions for the methyl radical CH 3 , of which ethane C 2 H 6 is a dimer. This error was corrected in by Carl Schorlemmer , who showed that the product of all these reactions was in fact ethane. At standard temperature and pressure, ethane is a colorless, odorless gas. Solid ethane exists in several modifications. In this form, the positions of the hydrogen atoms are not fixed; the molecules may rotate freely around the long axis. Cooling this ethane below ca. Rotating a molecular substructure about a twistable bond usually requires energy.
Gas laws. Which of the following instruments is used to measure Soil Water Tension?
Molar mass of Ethane C 2 H 6 is Then, lookup atomic weights for each element in periodic table : C: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by
Ethane molecular mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Witcher 3 iorveth
PbH 4. The physical origin of the barrier is still not completely settled, [16] although the overlap exchange repulsion [17] between the hydrogen atoms on opposing ends of the molecule is perhaps the strongest candidate, with the stabilizing effect of hyperconjugation on the staggered conformation contributing to the phenomenon. Y verify what is Y N? Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Binary compounds of hydrogen. This reaction proceeds through the propagation of the ethyl radical:. Henry's law constant k H. November 1, Modify the access date according your visit. Translate this page to Your Own Language. An object is placed between two inclined mirrors. University of Michigan. Railway Zone Wise Pages.
At standard temperature and pressure , ethane is a colorless, odorless gas.
See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. At this low temperature, gaseous methane can be separated from the liquefied ethane and heavier hydrocarbons by distillation. Ethane can be used as a refrigerant in cryogenic refrigeration systems. The Journal of Chemical Physics. Until they warm to room temperature, the vapors from liquid ethane are heavier than air and can flow along the floor or ground, gathering in low places; if the vapors encounter an ignition source, the chemical reaction can flash back to the source of ethane from which they evaporated. Henry's law constant k H. Retrieved Atmospheric ethane results from the Sun's photochemical action on methane gas, also present in these atmospheres: ultraviolet photons of shorter wavelengths than nm can photo-dissociate the methane molecule into a methyl radical and a hydrogen atom. PubChem Compound. Cooling this ethane below ca. PMID

Absolutely with you it agree. In it something is also to me it seems it is excellent idea. I agree with you.
Charming phrase
It agree, it is the amusing information