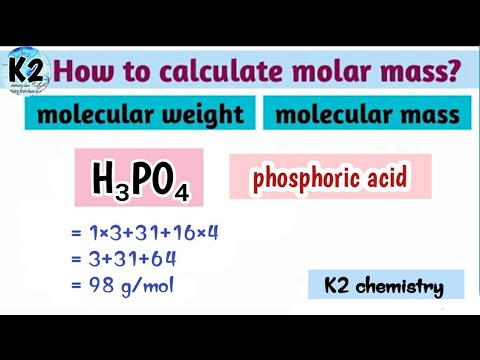
Equivalent weight of phosphoric acid
Incorporating a sensor with completely PFA perfluoroalkoxy wetted parts, size is optimized to the absolute limit. Employing a sensor with a sanitary structure, equivalent weight of phosphoric acid, using only PFA as the gme stock material. Being a chemically resistant sensor, it is capable of measuring various chemicals used in semiconductor processes. Concentration conversion is possible by inputting the relationship between chemical concentration and conductivity along with temperature characteristics.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In recent years, there has been a continuous increase in the incidence of urolithiasis, especially in highly developed countries. Therefore, the question arises which factors specific to these countries may be responsible for the increase in the incidence of this disease.
Equivalent weight of phosphoric acid
Aby znaleźć odpowiednią aplikację, należy użyć filtrów branż i próbek lub użyć wyszukiwania tekstowego. Możliwa jest dowolna kombinacja filtrów i wyszukiwania tekstowego. Należy pamiętać, że w wyniku wyszukiwania tekstowego uzyskuje się wyłącznie odpowiedzi, które zawierają dokładnie taki sam ciąg wyrazów jak podany w zapytaniu. Kliknij , aby uzyskać dalszą pomoc. Te sprawdzone w praktyce oraz odpowiednio przetestowane aplikacje pomogą w szybkim uzyskaniu dokładnych wyników pomiarów. Wyszukiwarka internetowa umożliwia przeszukiwanie bazy danych i znajdowanie aplikacji najlepiej dopasowanych do potrzeb. Our instruments provide consistent and accurate measurements and ensure compliance with international and regional regulations. Find below the regulations and standards our instruments comply with, for your respected industry. For more information on our digital density meters and refractometers , and how they compare to manual methods please see our Comparison of different measuring techniques. For density measurement guides, whitepapers, webinars, or more information about our products, please visit our Expertise Library.
The purpose of the analysis is to determine the dominant chemical form of the complexes for baseline 0 and in the presence of phosphoric acid at the tested concentrations 1, 2, equivalent weight of phosphoric acid, 3 and 4. This is due to the fact that the surface of the urinary stone is usually porous, which favours the adhesion of bacteria, which may become nucleation centres for subsequent layers of the urinary stone.
Use of this information is subject to copyright laws and may require the permission of the owner of the information, as described in the ECHA Legal Notice. EC number: CAS number: For the inhalation route there is no animal study available. Therefore, oral rat data is used to calculate a corresponding air concentration for humans and a route-to-route extrapolation for systemic effects is necessary to derive the correct starting point. In the case of oral-to-inhalation the inclusion of a default factor of 2 is recommended according to chapter R. According to Figure R. For the dermal route there is no animal study available.
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solid , and inorganic compound with the chemical formula H 3 P O 4. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Phosphoric acid forms esters , called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids ", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
Equivalent weight of phosphoric acid
Dear student, you haven't completed the reaction part, but don't worry I know about the reaction of H3PO4 with NaOH, this reaction is generally asked by students. We know that for the Acids, the equivalent weight is equal to the ratio of the molar mass and number of the hydrogen ions of the acid accepted by the base. We endeavor to keep you informed and help you choose the right Career path. When you look back in life , this app would have played a huge role in laying the foundation of your career decisions. Found everything I wanted and it solved all of my queries for which I was searching a lot A must visit No need to find colleges in other sites, this is the best site in India to know about any colleges in India. Get answers from students and experts Ask. Lal chandra 19th Oct, Answer later.
Worship music academy reviews
Phosphoric Acid. Provided by the Springer Nature SharedIt content-sharing initiative. Easy R The amount of added NaOH is shown in Table 8. The crystals shown in Fig. Szybkie linki Kontakt Wyszukiwarka Wyszukiwarka dokumentów Wyszukiwarka produktów Wyszukiwarka usług serwisowych Wideo. These spectrophotometric absorbance measurements are confirmed by microscopic observations Fig. Figure 5. For example, the absorbance value at pH 9 for baseline is about 0. Effect of antibiotic treatment on Oxalobacter formigenes colonization of the gut microbiome and urinary oxalate excretion Article Open access 12 August
Phosphoric acid is a colorless, odorless, inorganic compound. It is represented by the chemical formula H 3 PO 4.
At the same time, as the concentration of phosphoric acid increases, the growing struvite crystals are larger, which is disadvantageous because they are more difficult to remove from the urinary tract along with the urine. Arunkajohnsak, N. In a healthy person, there is a continuous flow of urine in the urinary tract, the person also ingests various fluids during the day and some of this urine is excreted. The results of spectrophotometric measurements are presented in Fig. On the basis of Table 6 it is possible to notice a slight decrease in the molar concentration of individual ions, resulting from the addition of an aqueous ammonia solution, i. Comments By submitting a comment you agree to abide by our Terms and Community Guidelines. Urology 73 , — The obtained results indicate that phosphoric acid present in artificial urine causes the nucleation of struvite to shift towards a lower pH, which means that struvite nucleates earlier in artificial urine compared to the control test. Seminarium internetowe na życzenie. Microorganisms can survive in already formed stone by building into its structure. Copy to clipboard. Sorry, a shareable link is not currently available for this article. Kliknij , aby uzyskać dalszą pomoc. You can also search for this author in PubMed Google Scholar.

It is remarkable, it is rather valuable phrase
The useful message