Elements chart with valency
Study chemistry with the searchable periodic table and quizzes.
Electrolysis is a process where electrical energy will change in chemical energy. The process happens in an electrolyte, a watery solution or a salt melting which gives the ions a possibility to transfer between two electrodes. The electrolyte is the connection between the two electrodes which are also connected to a direct current. This unit is called electrolyse cell and is shown in the picture below:. If you apply an electrical current, the positive ions migrate to the cathode while the negative ions will migrate to the anode.
Elements chart with valency
Link to the lesson. Nagranie dostępne na portalu epodreczniki. Oxygen compounds with other elements are called oxides. In all oxides, oxygen has a valency of two. On this basis, knowing the oxide formula, we can determine the valency of elements in oxides. The first term is the name of the element in the genitive and the second — the word oxide e. Many elements create several oxides in which their valency is different. An example is the compounds of lead with oxygen with the following molecular formulas: Pb O 2 and PbO. In the first oxide, lead has a valency of four, in the second - two. Therefore, to uniquely determine the type of compound, e. Oxygen valency is not determined in the names of oxides, since it is always two II. Note your conclusions. By creating the names of metal oxides belonging to groups 1st and 2nd, their valency is not given, because these elements always have only one characteristic valency in chemical compounds: metals from 1st group - one, from 2nd group — two.
An example is a molecule created by combining nitrogen and oxygen, the valency of which is two. SO trzy, 4.
.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The periodic table and Lewis diagrams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus contains 2 black dots, the second ring from the nucleus contains 8 black dots, and the third ring from the nucleus contains 7 red dots.
Elements chart with valency
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. Where you will get the HD images along with the explanation. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro. External links: Valence electrons of elements. Jay holds the roles of an author and editor at Periodic Table Guide, leveraging his ability to provide clear explanations on typically unexciting topics related to periodic table. He is passionate to help student, and he finds immense joy in his endeavors to make learning enjoyable and accessible.
Ashton wood nude
Na niebieskim tle napis Creation of a chemical compound name based on its total formula. Na białym tle duży niebieski napis en dwa o trzy, N2O3, dinitrogen trioxide. An example is the compounds of lead with oxygen with the following molecular formulas: Pb O 2 and PbO. For example, if you want to galvanize a piece of metal is used as cathode. I still have to repeat uzupełnij. By creating the names of metal oxides belonging to groups 1st and 2nd, their valency is not given, because these elements always have only one characteristic valency in chemical compounds: metals from 1st group - one, from 2nd group — two. The standard potential shows the capability, in regard to hydrogen ions, to give up electrons. You can find in this table see a fragment below the elements ordered by their standard potentials E 0. If the element belongs to the 1st group of the periodic table, its valency will be equal to one, if it is the element located in the 2nd group, it will have the valency equal to two. Following strong oxidants will be reduced have a negative standard potential and strong reducing agents have a high positive value. Oxygen in oxides has valency equal to two. Molecular formula.
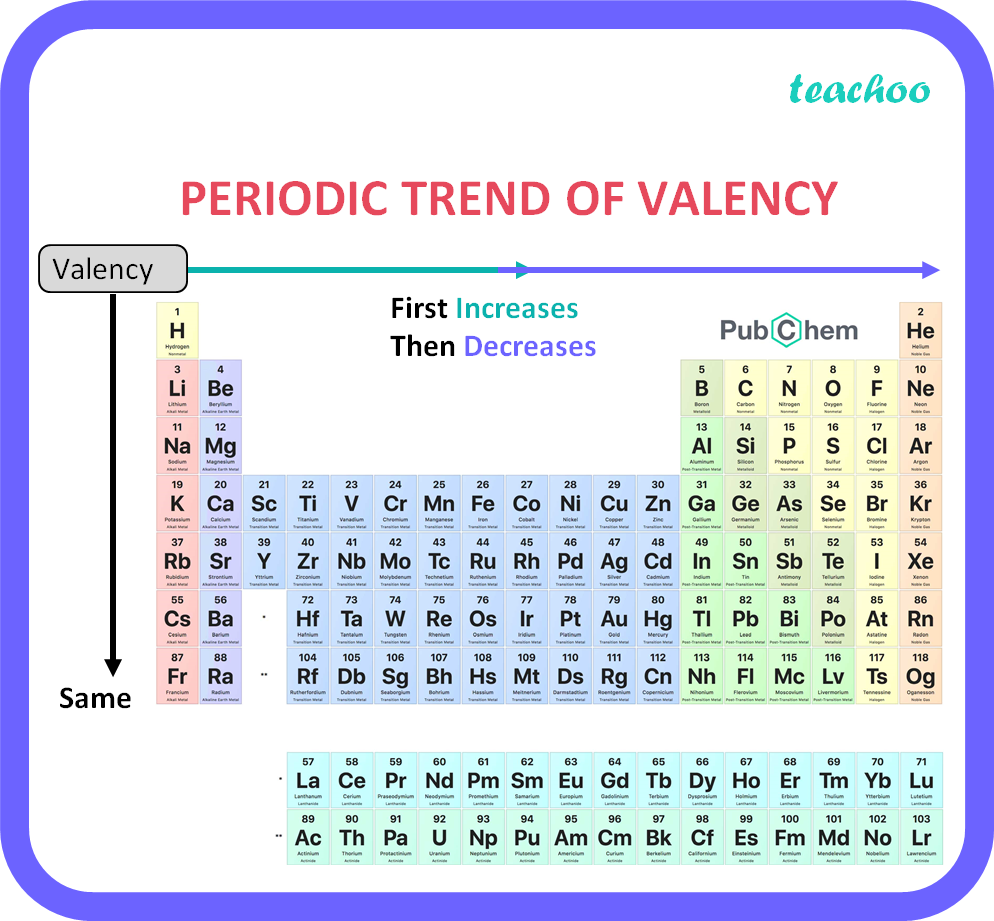
The valency of an element is a measure of its combining capacity and can be defined as. Oxidation State and valency are one of the most fundamental properties of elements and can be studied with the help of electron configurations. Electrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency or valence of an atom.
We write the molecular formula of barium oxide: BaO. Copyright © Lenntech B. Exercise 1. By creating the names of metal oxides belonging to groups 1st and 2nd, their valency is not given, because these elements always have only one characteristic valency in chemical compounds: metals from 1st group - one, from 2nd group — two. Remember that oxygen valency in these compounds is two. Electrolyse processes are difficult to control. Na białym tle duży niebieski napis en dwa o trzy, N2O3, dinitrogen trioxide. After determining the valency of nitrogen, which is three III , we can determine the name of the oxide. Valency of the element in the oxide. Indicate the answer showing the correct molecular formula of the chemical compound based on the systematic name - sulfur dioxide: Możliwe odpowiedzi: 1.

It agree, very useful message
I am sorry, that has interfered... At me a similar situation. Let's discuss.