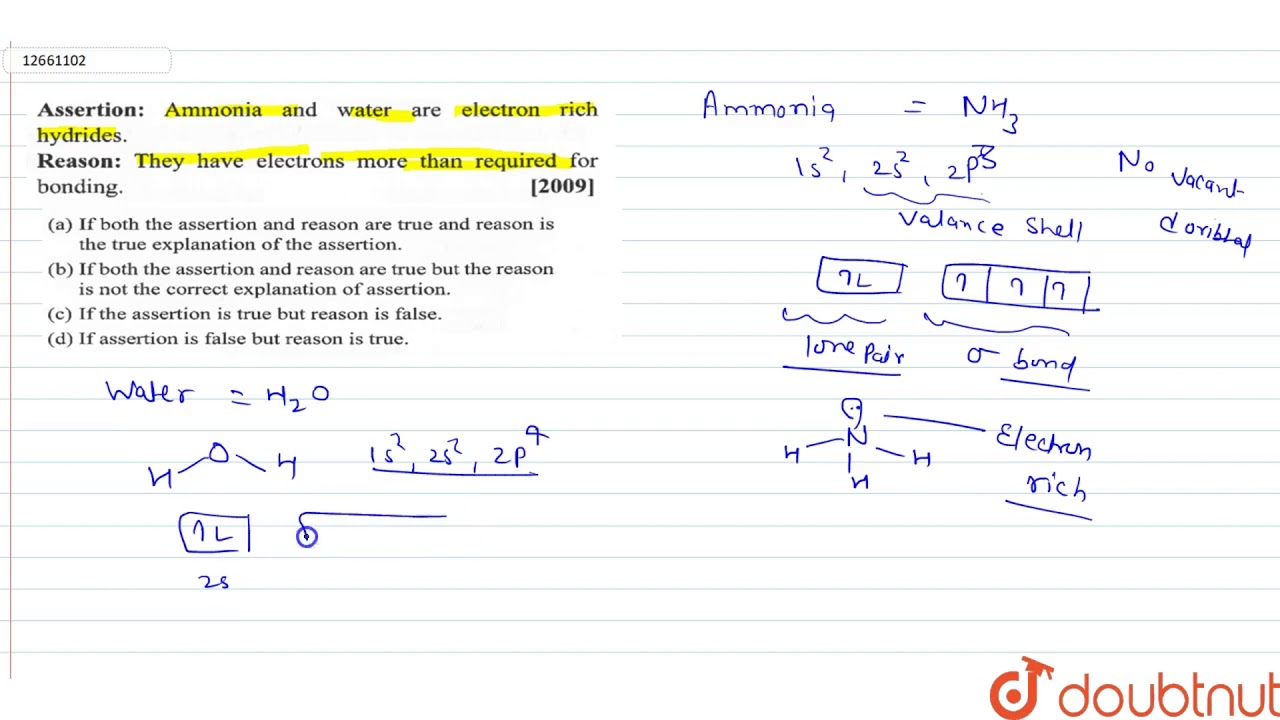
Electron rich hydrides
N H 3 is electron rich hydride. Describe electron-rich hydride. Which of the following hydrides is an electron-rich hydride?
Hydrides - Hydrides are the binary compound of hydrogen with other elements. Let 'X" be any element, then -. Ex - MgH 2 is the hydride of Magnesium. Based upon the number of electrons and bond type covalent or molecular hydrides are classified into -. So, match each hydrides accordingly we get, a - iv , b - i , c - ii , d - iii. Last updated on Jan 2, Get Started.
Electron rich hydrides
Define the following terms: a electron-deficient b electron - precise c electron-rich compound. Justify your answer with an example? Example: CH 4 , SiH 4 , etc. What do you understand by i electron-deficient, ii electron-precise, and iii electron-rich compounds of hydrogen? Provide justification with suitable examples. Byju's Answer. Open in App. Electron deficient: Electron-deficient compounds are those compounds that don't have a sufficient number of electrons to complete the octet of the central atom. These compounds contain insufficient numbers of electrons to form normal electron-pair bonds between each pair of bonded atoms. Electron deficient compounds exist in polymorphic forms to complete their electron deficiency.
ESIC Stenographer.
.
Hydride , in simple terms, is said to be the anion of hydrogen. It is a chemical compound where the hydrogen atoms exhibit nucleophilic, basic or reducing properties. Compounds of hydrogen with less electronegative elements are known as hydrides. So, when hydrogen reacts with any other element, the product formed is considered to be a hydride. If we closely observe the periodic table, hydride formation is not seen from VA group elements, and this condition is known as the hydride gap.
Electron rich hydrides
The combination of hydrogen with another element produces a hydride, E x H y. The formal charge or oxidation state of the hydrogen in these compounds is dependant on the relative electronegativity of the element in question. Hydrogen compounds with highly electropositive metals, i. As a consequence, in the solid state the hydride ion replicates that of a halide ion e. Unlike the halide ions that are soluble in water, the hydride ion reacts with water, 2. The liberation of hydrogen was used as a commercial source of hydrogen for small-scale applications. Group 1 and 2 metal hydrides can ignite in air, especially upon contact with water to release hydrogen, which is also flammable. Hydrolysis converts the hydride into the analogous hydroxide, which are caustic bases. In practice, most ionic hydrides are dispensed as a dispersion in oil, which can be safely handled in air.
Army promotion cutoff
NCL Staff Nurse. RCFL Technician. Punjab Superior Judicial Service. Telangana High Court Typist. Bihar ANM. Rajasthan Pre D. Uttarakhand D. Punjab Civil Service. Telangana High Court Examiner. Gujarat Police ASI.
Not only the metal hydrides are needed as synthetic reagents for preparing the transition metal organometallic compounds but they also are required for important hydride insertion steps in many catalytic processes. The first transition metal hydride compound was reported by W.
Suggested Exams. Rajasthan GNM. Navy AA. IB Security Assistant. NHB Assistant Manager. UP Police Constable. Rajasthan Computer Teacher. SSB Constable. Punjab Superior Judicial Service. Rajasthan High Court Clerk.

Excuse for that I interfere � To me this situation is familiar. I invite to discussion. Write here or in PM.