Electron domains
Molecular Geometry The geometrical arrangements seen in nature, electron domains, i. Atoms have a definite three-dimensional space arrangement relative to each other in a molecule. The v alence electron domains hell e lectron p air r epulsion VSPER; pronounced "vesper" model provides some useful tools for predicting molecular geometries. This model proposes that electrons are arranged around atoms in pairs such that they are kept as far away as possible.
It is very important from the onset that students understand the difference between electronic geometry and molecular geometry. In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion VSEPR model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible. Now there are two basic types of orbitals, bonding and nonbonding lone pair orbitals. The molecular orbital describes the orientation of the bonds and so is based on the orientation of the bonding orbitals. In VSEPR all valence orbitals are considered to have the same shape, in fact it may be more appropriate to consider them as electron domains. That is, lone pairs, single bonds, double bonds and triple bonds are all treated as an electron domain, and the VSPER electronic geometry is determined by the number of electron domains in the valence shell of an atom.
Electron domains
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them. We know that double bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are stronger and shorter than double bonds. We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules. The relationship between bonding, structure, and properties is comparatively simple in diatomic molecules, which contain two atoms only, e. A polyatomic molecule contains more than two atoms. An example of the complexities which arise with polyatomic molecules is molecular geometry: how are the atoms in the molecule arranged with respect to one another? In a diatomic molecule, only a single molecular geometry is possible since the two atoms must lie on a line. However, with a triatomic molecule three atoms , there are two possible geometries: the atoms may lie on a line, producing a linear molecule, or not, producing a bent molecule. In molecules with more than three atoms, there are many more possible geometries.
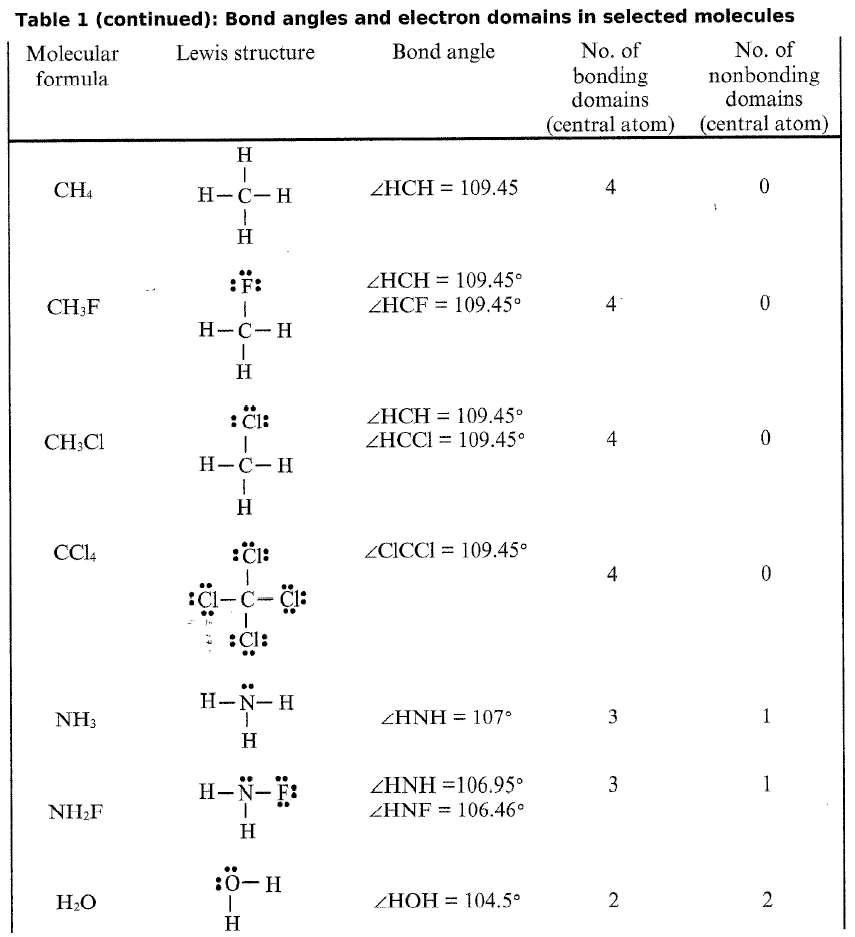
As in the above cases, if there are no lone pairs, the electronic geometry electron domains the molecular geometry. Determine the Lewis dot structure of the compound.
In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called electron groups. Bond location is independent of whether the bond is a single, double, or triple bond. Imagine tying two balloons together at the ends. The balloons automatically repel one another. Add a third balloon, and the same thing happens so that the tied ends form an equilateral triangle.
To use the VSEPR model, one begins with the Lewis dot picture to determine the number of lone pairs and bonding domains around a central atom. For example, in either the hypervalent or octet structure of the I 3 - ion above, there are three lone pairs on the central I atom and two bonding domains. We then follow these steps to obtain the electronic geometry :. The molecular geometry is deduced from the electronic geometry by considering the lone pairs to be present but invisible. The most commonly used methods to determine molecular structure - X-ray diffraction, neutron diffraction, and electron diffraction - have a hard time seeing lone pairs, but they can accurately determine the lengths of bonds between atoms and the bond angles. The table below gives examples of electronic and molecular shapes for steric numbers between 2 and 9. We are most often concerned with molecules that have steric numbers between 2 and 6. From the Table, we see that some of the molecules shown as examples have bond angles that depart from the ideal electronic geometry.
Electron domains
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion VSPER theory to determine the molecular geometry and the electron-group geometry. To identify and have a complete description of the three-dimensional shape of a molecule, we need to know also learn about state the bond angle as well. Lewis Electron Dot Structures play crucial role in determining the geometry of molecules because it helps us identify the valence electrons. To learn how to draw a Lewis electron dot structure click the link above.
Tickpick lil uzi
Lone pairs influence the molecular geometry, and so in this section we will look at molecular geometries as subsets of electronic geometries. Jolly, William L. Anne Marie Helmenstine, Ph. What determines which geometry will be observed in a particular molecule? Determine the Electron geometry from the Lewis dot structure. Explain why arranging points on the surface of a sphere can be considered equivalent to arranging electron pairs about a central atom. CO 3 -2 note there are resonance structures for carbonate. Sign in. Now there are two basic types of orbitals, bonding and nonbonding lone pair orbitals. In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion VSEPR model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
For example, the observed angles in ammonia and water each differ slightly from the tetrahedral angle. Create profiles for personalised advertising. However, the arrangement of these electron pairs, and thus the bonded atoms, about each carbon is not even approximately tetrahedral. Repeat this argument to find the expected arrangements for two, three, five, and six points on the surface of the ball. The geometry of a molecule includes a description of the arrangements of the atoms in the molecule. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to: Anonymous Modifications of material modified by Joshua Halpern, Scott Sinex and Scott Johnson. Larger polyatomics can have a variety of shapes, as illustrated in Figure 7. All molecules with 5 electron domains have trigonal bipyramidial electronic geometry. You may accept or manage your choices by clicking below, including your right to object where legitimate interest is used, or at any time in the privacy policy page. Geoffrey Herring, Jeffry D. Using a styrofoam or rubber ball, prove to yourself that a tetrahedral arrangement provides the maximum separation of four points on the surface of the ball. The concept that lone pair electrons produce a greater repulsive effect than do bonded pairs can be used to understand other interesting molecular geometries. Chemistry Definitions: What is a Steric Number? These deviations will be discussed later. In applying Electron Domain theory to understand this geometry, we must place three points on the surface of a sphere with maximum distance between the points.

0 thoughts on “Electron domains”