Einstein photoelectric effect class 12
Read till the end to know more. In this article, we will understand how Einstein explained the photoelectric effect and why earlier scientists failed to do this. This concept came into the picture in the year by Heinrich Hertz. It was further researched and observed by Lenard in
The photoelectric effect is a phenomenon where electrons are emitted from the metal surface when light of sufficient frequency is incident upon it. The concept of the photoelectric effect was first documented in by Heinrich Hertz and later by Lenard in Hertz who had proved the wave theory himself did not pursue the matter as he felt sure that it could be explained by the wave theory. However, the concept failed in the following accounts:. Electrons emitted from underneath the metal surface lose some kinetic energy during the collision. But the surface electrons carry all the kinetic energy imparted by the photon and have the maximum kinetic energy. Below this frequency, there is no electron emission.
Einstein photoelectric effect class 12
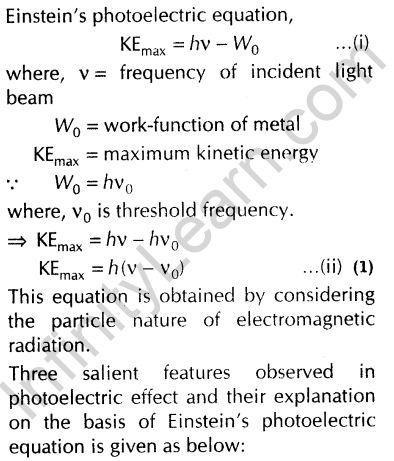
Einsteins photoelectric equation is. Write Einstein's photoelectric equation. What is photoelectric effect? Mention the Einstein's photoelectric equation. Explain the laws of photoelectric effect from the Einstein photoelectric equation. By photoelectric effect, Einstein, proved. Define the threshold frequency v 0 and stopping potential V 0. Write Einsteins's photoelectric equation. Explain the terms i threshold frequency, and ii stopping potential. Einstein's work on photoelectric effect gives support to. What is the photoelectric effect? Frame Einstein. The threshold frequency of a photoelectric effect depends on. Derive the relation for Einstein.
Which metals exhibit photoelectric effect? Using this, we have. The Zeroth law of thermodynamics states that any system which is isolated from the rest will evolve so as to maximize its own internal energy.
Albert Einstein, a German physicist, is considered one of the greatest scientists of all times. He has contributed to the fields of general relativity , black holes, photoelectric effect, to name a few. In , Einstein was awarded the Nobel Prize in physics for the discovery of the photoelectric effect. Einstein's idea about light was revolutionary and magnificent. He gave an efficient method of irradiation. Light has some tiny group of particles known as photons. These particles consist of higher energy, which is also called the quantum of radiation.
The photoelectric effect is a phenomenon where electrons are emitted from the metal surface when light of sufficient frequency is incident upon it. The concept of the photoelectric effect was first documented in by Heinrich Hertz and later by Lenard in Hertz who had proved the wave theory himself did not pursue the matter as he felt sure that it could be explained by the wave theory. However, the concept failed in the following accounts:. Electrons emitted from underneath the metal surface lose some kinetic energy during the collision. But the surface electrons carry all the kinetic energy imparted by the photon and have the maximum kinetic energy. Below this frequency, there is no electron emission. Thus, the energy of a photon with this frequency must be the work function of the metal.
Einstein photoelectric effect class 12
In , the annus mirabilis miracle year of Physics, Albert Einstein proposed an equation to explain this effect. Einstein argued that light was a wave that interacts with matter in the form of a packet of energy or a quantum of energy. He proposed a weird but effective model of radiation.
Business development manager sueldo
Did not receive OTP? If the electron gets energy equal to threshold frequency, its kinetic energy becomes zero after coming out of the surface. He found out that the radiation from different gaseous atoms consisted of a different set of wavelengths. Who initially explained the photoelectric effect? What is the photoelectric equation given by Einstein? However, the electrons present on the top layer of the metal absorb all the kinetic energy transferred by a photon. Zener diode Learn about the basics, applications, working, and basics of the zener diode. An electron cannot emerge from an object when the photon energy is too low. It is named after Thomas Young. V max is the maximum kinetic energy of the electron. Because non conductive oxide ports on metal surfaces raise the energy barrier in image extraction, many functional investigations and devices based on the effect of electric photography require clean metal surfaces in the extracted tubes. Explain the laws of photoelectric effect from the Einstein photoelectric equation. Also, it is essential to note that Niel Bohr contributed significantly to the development of the photoelectric effect. Also, v0 is the Stopping Potential, So.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Albert Einstein, a German physicist, is considered one of the greatest scientists of all times. At low pressure, it was discovered that radiation from gaseous atoms contained a set of different wavelengths. Related articles. The photoelectric effect is a phenomenon where electrons are emitted from the metal surface when light of sufficient frequency is incident upon it. However, the electrons present on the top layer of the metal absorb all the kinetic energy transferred by a photon. The remaining energy of the photon forms a free negative charge called photoelectron. Hertz who had proved the wave theory himself did not pursue the matter as he felt sure that it could be explained by the wave theory. FREE Signup. Frequently asked questions. Zeroth law of Thermodynamics The Zeroth law of thermodynamics states that any system which is isolated from the rest will evolve so as to maximize its own internal energy. Because increasing the intensity of low-intensity light will only increase the number of low-energy photons, no single photon with sufficient electron output will be produced. What is threshold frequency in photoelectricity? Heat Energy Definition. From the experiments of the Photoelectric effect, it is found that no electron emission occurs if the incident radiation has a frequency less than the threshold frequency. The energy transferred to every light particle, quanta or photon depends on the frequency v of light, shown by the photoelectric effect equation.

I join. It was and with me. Let's discuss this question. Here or in PM.