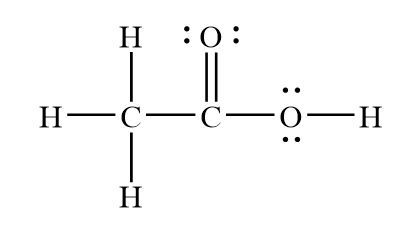
Draw the lewis structure for acetic acid
There are 2 lone pairs on both the Oxygen atoms O.
Cheers for this great post. I believe that you have raised some interesting poinst here. Keep up the excellent blog. Created by MakeTheBrainHappy. You could alternatively also draw the structure by including two dots for every bond. As you can see every single element has a filled valence shell with the two oxygen's each containing two lone pairs of electrons, the only instance of this phenomena within the Lewis Structure.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. Place the remaining six valence electrons around the oxygen atoms in pairs. One oxygen will have two lone pairs and two bonding pairs of electrons in the atom attached to hydrogen. The remaining oxygen bonded to carbon will have three lone pairs and one bonding pair of electrons. Lone pairs are non-bonding pairs of electrons. Each hydrogen atom has two electrons around it. The formal charge is a measure of the distribution of electrons in a molecule.
These are again elevated due to the polarity of acetic acid.
.
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. But as per the rule, we have to keep hydrogen outside. So, carbon which is less electronegative than oxygen should be placed in the center and the remaining hydrogen atoms as well as COOH group will surround it.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen.
Colour shampoo chemist warehouse
The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. These are again elevated due to the polarity of acetic acid. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. Keep up the excellent blog. Carbon is group 14 element on the periodic table. To check the formal charges in water, we use the following formula:. Now to make this carbon atom stable, you have to shift the electron pair from the outer oxygen atom so that the carbon atom can have 8 electrons i. Facebook messenger. One oxygen will have two lone pairs and two bonding pairs of electrons in the atom attached to hydrogen. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. Labels: chemistry , Lewis Structures , Science , zlatest. It is calculated by subtracting the number of non-bonding electrons and half of the bonding electrons from the number of valence electrons in an atom.
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond.
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. It is frequently utilized as a polar solvent in lab research settings and thereby has been involved in certain medical practices. Acetic acid is useful due to its properties as a polar solvent and building block for other molecules, containing both a methyl CH3 and COOH functional group. Leave a Comment Cancel Reply Your email address will not be published. Oxygen is group 16 element on the periodic table. It has only 6 electrons and it is unstable. In other way you can also see that the carbon atom is surrounded with three hydrogen atoms and one COOH group. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. In a sense this is a modified structure of methane CH4 with the replacement of one hydrogen with the replacement of a carboxylic group -COOH. You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons.

It agree, rather useful piece