Do ionic compounds dissolve in water
We have learned that solutions can be formed in a variety of combinations using solids, do ionic compounds dissolve in water, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, and yet vinegar and water will?
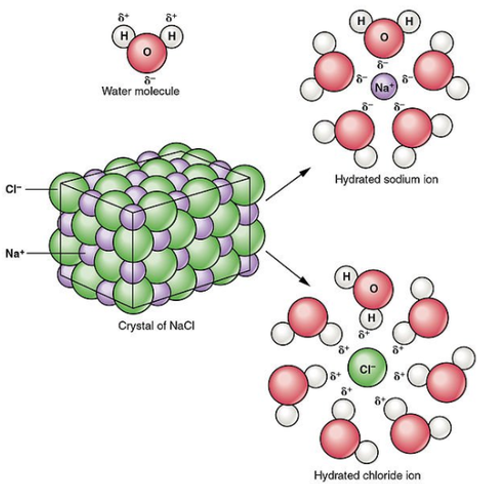
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion. Ionic compounds dissolve in water due to the difference between its lattice energy and its hydration energy.
Do ionic compounds dissolve in water
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution of ionic compounds in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them.
Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons.
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining. The resultant ionic solution becomes an electrolyte, which means it can conduct electricity. By virtue of the arrangement of the hydrogen atoms around the oxygen, each water molecule carries a polar charge.
Water-soluble ionic compounds such as acids, bases, and salts dissociate in water forming ions. In fact, this interaction between the ions and water molecules is the driving force for dissolving and ionizing the salt. The first thing you will need is to determine whether the compound is soluble or not. If it is not, then you cannot write a dissociation equation for it because it only dissociates when dissolved in water. To determine this, use the following rules for different combinations of cations and anions that make the salt:. The perchlorate ion ClO 4 — is listed as soluble with any cation without exceptions.
Do ionic compounds dissolve in water
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining.
Hasta kabul kursu kaldirildimi
These substances constitute an important class of compounds called electrolytes. A red sphere in one of these clusters is labeled O. All phosphates are insoluble, so Sr 3 PO 4 2 is insoluble. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, the substance is a weak electrolyte does not conduct electricity as well. If solutions of sodium nitrate and ammonium chloride are mixed, no reaction occurs. Question aa. The solubility product constant K sp measures this equilibrium point. Impact of this question views around the world. Immiscible - Liquids that do not have the ability to dissolve in each other. This process represents a physical change known as dissociation. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Austin State University with contributing authors. This maximum amount is specified as the solubility of the solute. This occurs when the positive cation from the ionic solid is attracted to the negative end of the water molecule oxygen and the negative anion of the ionic solid is attracted to the positive end of the water molecule hydrogen. The solubility of a solid in water increases with an increase in temperature.
The substances described in the preceding discussion are composed of molecules that are electrically neutral; that is, the number of positively-charged protons in the nucleus is equal to the number of negatively-charged electrons. In contrast, ions are atoms or assemblies of atoms that have a net electrical charge.
If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Therefore, the solubility of a gas decreases as the temperature increases. Search site Search Search. Every ionic compound has an energy holding the lattice structure of the compound, known as lattice energy. Let us consider what happens at the microscopic level when we add solid KCl to water. The resultant ionic solution becomes an electrolyte, which means it can conduct electricity. One of the K superscript plus purple spheres is surrounded by four of the red and white clusters. These ions are so vital to metabolism that they must be replenished when the body dehydrates through exercise or sickness. This is why athletes prefer electrolytic drinks to pure water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them.

It is grateful for the help in this question how I can thank you?