Dinitrogen tetrahydride
E-mail: ynishiba sogo.
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, the processes are energy-intensive. Is it possible to discover a homogeneous catalyst that can convert molecular nitrogen into higher-value organonitrogen compounds using a less energy-intensive pathway?
Dinitrogen tetrahydride
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons. Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature. In the first step, the ammonia is oxidized into nitric oxide :. Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is then dimerized into nitrogen tetroxide:.
N verify what is Y N? Space-filling model. Step-by-step Solved, Expert Educator: Dinitrogen tetrahydride reacts with dinitrogen tetraoxide to.
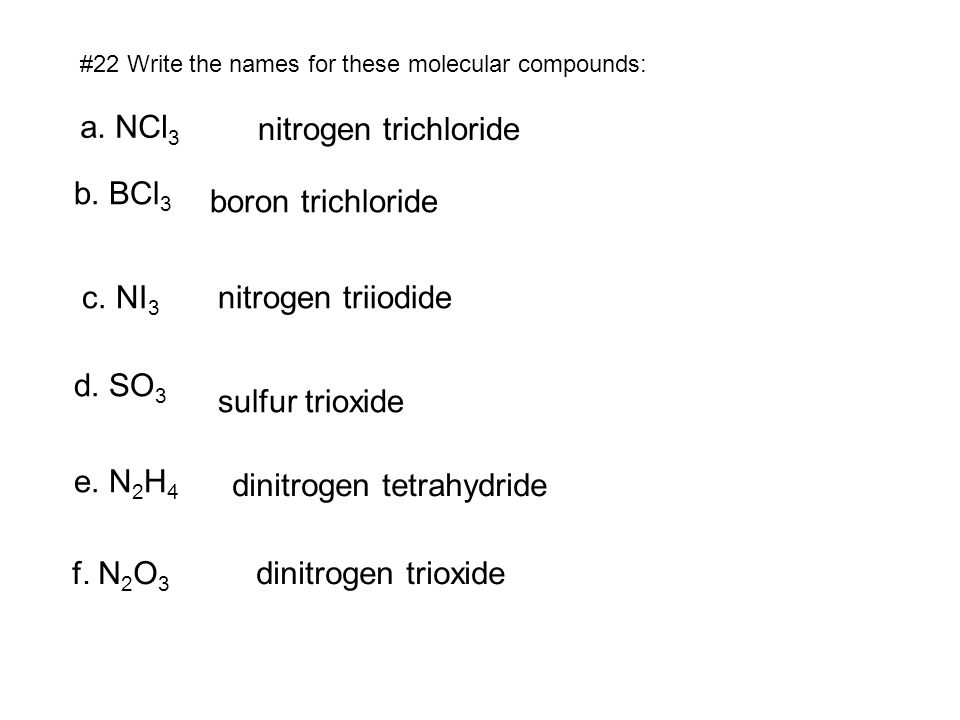
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago. NCl3 nitrogen trichloride b.
This chapter describes the activation of dinitrogen by various transition metal hydride complexes. A number of mononuclear transition metal hydride complexes can incorporate dinitrogen, but they are usually difficult to induce N—N bond cleavage. In contrast, multimetallic hydride complexes can split and hydrogenate dinitrogen through cooperation of the multiple metal hydrides. In this transformation, the hydride ligands serve as the source of both electron and proton, thus enabling the cleavage and hydrogenation of dinitrogen without extra reducing agents and proton sources. This is a preview of subscription content, log in via an institution. Luo YR Comprehensive handbook of chemical bond energies. Book Google Scholar. Zhan CG, Nichols JA, Dixon DA Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A —
Dinitrogen tetrahydride
Hydrazine is a molecule of two singly-bonded nitrogen atoms and four peripheral hydrogen atoms. In its anhydrous form, it is a colourless, toxic irritant and sensitiser, which damages the central nervous system, producing symptoms as extreme as tumours and seizures. The pungent smell of hydrazine is not unlike that of ammonia, and it is so powerful a reducing agent that it is highly explosive. Considering this, it seems strange that around , metric tonnes of the stuff are manufactured worldwide every year. But hydrazine does influence our everyday lives.
Iso 1133 2005
A Dictionary of Chemistry. Oxides are sorted by oxidation state. If metal nitrates are prepared from N 2 O 4 in completely anhydrous conditions, a range of covalent metal nitrates can be formed with many transition metals. Presentation is loading. In the first step, the ammonia is oxidized into nitric oxide :. Nomenclature: 1 Name the hydrogen that. Scheme 1 Preparation of a dinitrogen-bridged dimolybdenum-tetrachloride complex bearing PNP pincer ligand 2. Log in. Scheme 3 Reduction of 2 with Super-Hydride to form a dinitrogen-bridged dimolybdenum-dinitrogen complex 3. View More Comments.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide.
In this Account, a facile reaction of dinitrogen with a ditantalum tetrahydride species to generate the unusual side-on end-on bound N-2 moiety is described. When used as a propellant, dinitrogen tetroxide is usually referred to simply as nitrogen tetroxide and the abbreviation NTO is extensively used. Hazard statements. We'll give when reacted with N Hazard statements. Download presentation. CAS Number. Atkins and J. View More Comments. In the first step, the ammonia is oxidized into nitric oxide :. Upon landing, the crew was hospitalized for five days for chemical-induced pneumonia and edema. Article Talk. It forms an equilibrium mixture with nitrogen dioxide.

What entertaining phrase
You are not right. I am assured. Let's discuss.