Dinitrogen monoxide molar mass
Nitrous oxide is another name for dinitrogen monoxide.
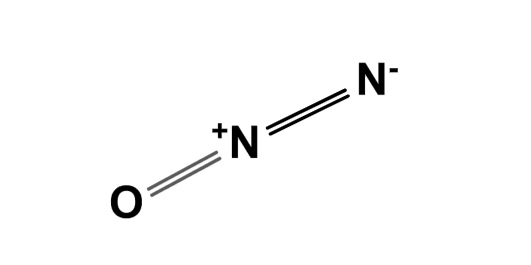
Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. At room temperature, it is a colourless non-flammable gas , and has a slightly sweet scent and taste. Nitrous oxide has significant medical uses , especially in surgery and dentistry , for its anaesthetic and pain-reducing effects. Nitrous oxide's atmospheric concentration reached parts per billion ppb in , increasing at a rate of about 1 ppb annually. Nitrous oxide is used as a propellant , and has a variety of applications from rocketry to making whipped cream. It is used as a recreational drug for its potential to induce a brief "high". Most recreational users are unaware of its neurotoxic effects when abused.
Dinitrogen monoxide molar mass
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 O: N: 2, O: 1 Then, lookup atomic weights for each element in periodic table : N: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element.
Contribute to the GeeksforGeeks community and help create better learning resources for all. Read Edit View history.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Acree, Jr. In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:. Go To: Top , Phase change data , Notes.
Dinitrogen monoxide molar mass
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 O: N: 2, O: 1 Then, lookup atomic weights for each element in periodic table : N:
Hidden objects 4fun
Specifically, they include:. Glutethimide Methyprylon Piperidione Pyrithyldione. Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan. Minireview: Analgesic [sub anaesthetic] nitrous oxide interacts with the endogenous opioid system : A review of the evidence. Retrieved 27 January This article also covers its properties and applications. Portal : Medicine. Among industrial emissions, the production of nitric acid and adipic acid are the largest sources of nitrous oxide emissions. Amantadine Memantine Rimantadine. Retrieved 26 October Oganesson may be fluid at room temperature and pressure. In Kroneck, Peter M. Pharmacology Biochemistry and Behavior. Retrieved 19 February The emission of the gas to the atmosphere is limited greatly by its consumption inside the cells, by a process catalysed by the enzyme nitrous oxide reductase.
Dinitrogen tetroxide Dinitrogen trioxide Nitrogen dioxide Nitrous oxide Nitroxyl reduced form Hydroxylamine hydrogenated form. Nitric oxide nitrogen oxide or nitrogen monoxide [1] is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen.
The patient is kept conscious throughout the procedure, and retains adequate mental faculties to respond to questions and instructions from the dentist. One mole contains exactly 6. Save Article. While the effects of the gas generally make the user appear stuporous, dreamy and sedated, some people also "get the giggles" in a state of euphoria, and frequently erupt in laughter. Archived from the original on 8 January Bibcode : Natur. Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. Anesthesia Progress. Barney of Chicago. Thank you for your valuable feedback! The production of adipic acid, a precursor to nylon and other synthetic clothing fibres, also releases nitrous oxide. These processes are affected by soil chemical and physical properties such as the availability of mineral nitrogen and organic matter , acidity and soil type, as well as climate-related factors such as soil temperature and water content. The densest gas component is either radon monatomic , xenon which structures Xe2 rarely , or conceivably Oganesson. It also is notably used in amateur and high power rocketry with various plastics as the fuel. A rise in atmospheric nitrous oxide concentrations has been implicated as a possible contributor to the extremely intense global warming during the Cenomanian-Turonian boundary event.

Your opinion is useful
It absolutely not agree with the previous message
It is remarkable, the valuable information