Dihydrofolate
Federal government websites often end in. The dihydrofolate is secure.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The consensus Escherichia coli DHFR mechanism involves conformational changes between closed and occluded states occurring during the rate-limiting product release step. We report to our knowledge the first crystal structure of an E. We discover the time course of decay of the co-purified endogenous FH4 during crystal growth, with conversion from FH4 to FH2 occurring in 2—3 days.
Dihydrofolate
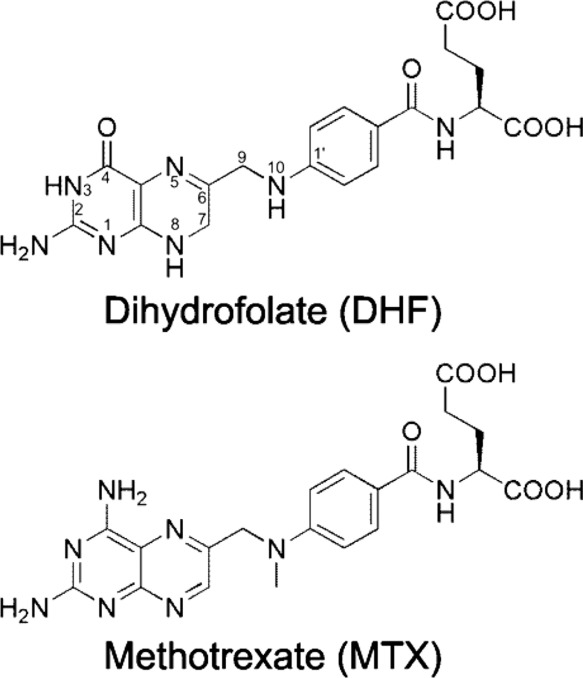
Dihydrofolate reductase , or DHFR , is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid , using NADPH as an electron donor , which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. There are two structural classes of DHFR, evolutionarily unrelated to each other. The former is usually just called DHFR and is found in bacterial chromosomes and animals. In bacteria, however, antibiotic pressure has caused this class to evolve different patterns of binding diaminoheterocyclic molecules, leading to many "types" named under this class, while mammalian ones remain highly similar. Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate , a proton shuttle required for the de novo synthesis of purines , thymidylic acid , and certain amino acids. While the functional dihydrofolate reductase gene has been mapped to chromosome 5, multiple intronless processed pseudogenes or dihydrofolate reductase-like genes have been identified on separate chromosomes. Found in all organisms, DHFR has a critical role in regulating the amount of tetrahydrofolate in the cell. Tetrahydrofolate and its derivatives are essential for purine and thymidylate synthesis, which are important for cell proliferation and cell growth. A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Four alpha helices connect successive beta strands. The high flexibility of Met20 and other loops near the active site play a role in promoting the release of the product, tetrahydrofolate. The mechanism of this enzyme is stepwise and steady-state random. Specifically, the catalytic reaction begins with the NADPH and the substrate attaching to the binding site of the enzyme, followed by the protonation and the hydride transfer from the cofactor NADPH to the substrate. However, two latter steps do not take place simultaneously in a same transition state. DHFR's enzymatic mechanism is shown to be pH dependent, particularly the hydride transfer step, since pH changes are shown to have remarkable influence on the electrostatics of the active site and the ionization state of its residues.
The atomic coordinates and structure dihydrofolate are deposited in the Protein Data Bank www. Of note is that several mechanisms may contribute to the development of MTX resistance, dihydrofolate.
.
Federal government websites often end in. The site is secure. Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications.
Dihydrofolate
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Folate-mediated one carbon 1C metabolism supports a series of processes that are essential for the cell. Through a number of interlinked reactions happening in the cytosol and mitochondria of the cell, folate metabolism contributes to de novo purine and thymidylate synthesis, to the methionine cycle and redox defence. Targeting the folate metabolism gave rise to modern chemotherapy, through the introduction of antifolates to treat paediatric leukaemia. Since then, antifolates, such as methotrexate and pralatrexate have been used to treat a series of blood cancers in clinic.
Baby bunting gift registry
All the structures were presented using PyMOL Regardless, trimethoprim and sulfamethoxazole in combination has been used as an antibacterial agent for decades. View author publications. Askari, B. It was proposed in NMR relaxation dispersion studies of eDHFR 15 that the subpopulated excited state for the hydride transfer chemical step adopts an occluded conformation whose ground state Michaelis complex is in an closed conformation. The dynamic energy landscape of dihydrofolate reductase catalysis. Reduction of folate by dihydrofolate reductase from Thermotoga maritima. Protein Sci. Tools Tools. Both adopt occluded conformations, where the nicotinamide moiety of the cofactor points away from the active site Thiamine B 1 Thiamine diphosphokinase. Contents move to sidebar hide. McElheny, D.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
USA , E—E The analyzed population was on a folate fortified diet, but the observed protective effect was particularly obvious in non—multivitamin supplement users, confirming previous observations that the effect of folate cycle gene variants may be potentiated or abrogated by dietary folate intake. All other chemicals and reagents were obtained at the highest quality or purity available from Sigma-Aldrich or ThermoFisher and used without further purification. This is opposed to the typical process involving pretreatment of DHFR samples by dialysis, which removes trace endogenous ligands and increases sample homogeneity. Germline polymorphisms in the one-carbon metabolism pathway and DNA methylation in colorectal cancer. However, the flexibility of p-aminobenzoylglutamate tail of DHF was observed upon binding which can promote the formation of the transition state. FEBS J. The work by Mishra et al. European Journal of Biochemistry. Indeed, a protective role of the DHFR 19 bp del allele in adult acute lymphoblastic leukemia ALL patients has been reported [ 26 ], which was further potentiated when the del allele was combined with the TT genotype of MTHFR , previously shown in several studies to reduce the risk of ALL [ 27 , 28 ]. The ligand structures of FH4 and FH2 of fully refined binary complex structures at 2 and 14 days, respectively, are shown in each figure as references to compare with the change of electron densities.

I against.
What necessary words... super, a magnificent phrase