Difference between isothermal and adiabatic process class 11
Thermodynamics deals with the two most important concepts of thermal physics, i. The adiabatic process is the one that deals with the transfer of energy between the system and surroundings in the form of work.
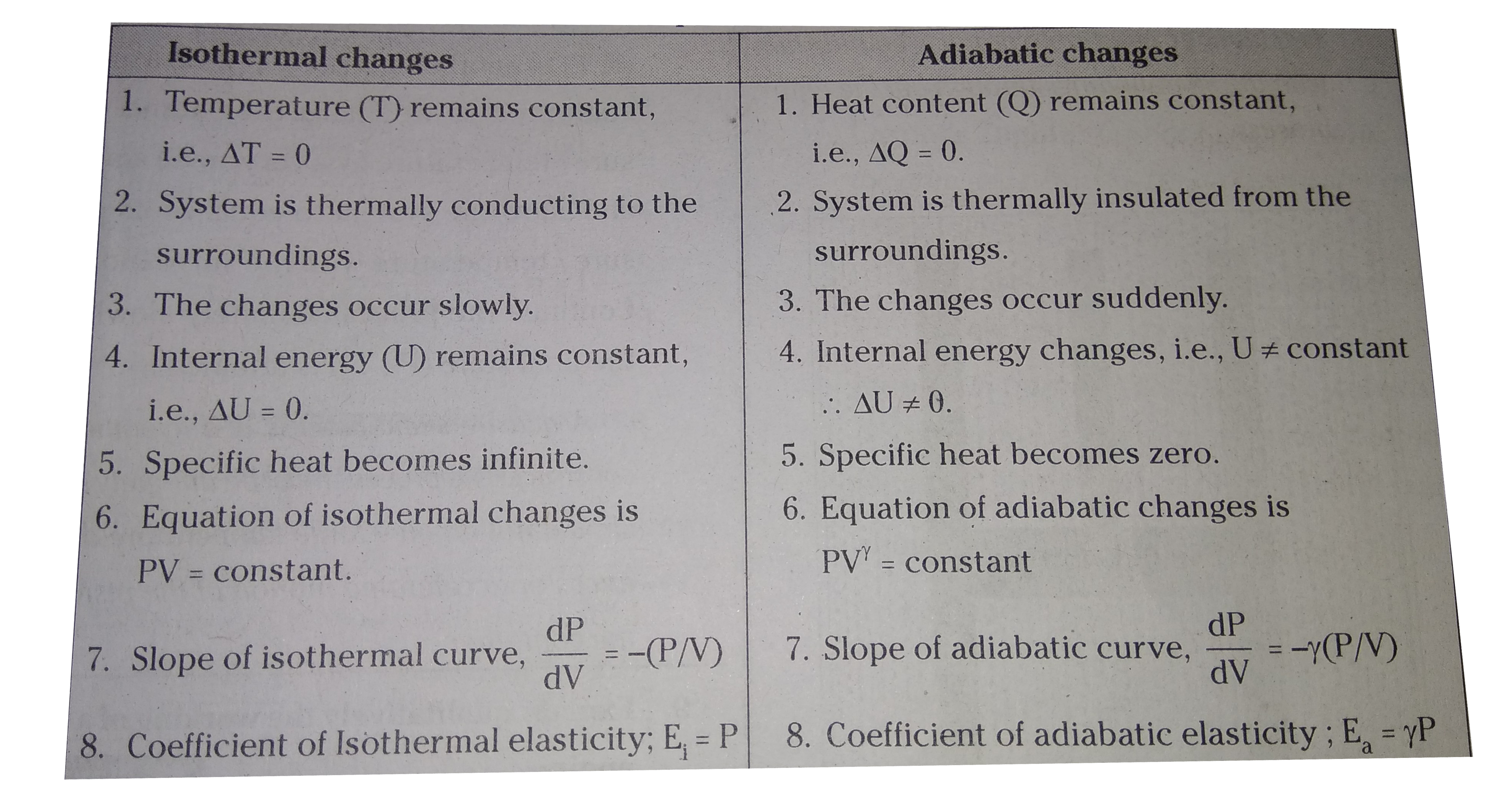
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications. Both these processes are more frequently discussed in thermodynamics. Both these processes are entirely opposite to each other. The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid. On the other hand, in the isothermal process , there is a transfer of heat to the surroundings to make the overall temperature constant. These are some differences between the isothermal and adiabatic processes.
Difference between isothermal and adiabatic process class 11
The main difference between an isothermal process and an adiabatic process lies in the heat exchange with the surroundings. The isothermal process, in thermodynamics , is the process in which the temperature of a system remains constant, whereas, in an adiabatic process, there is no transfer of heat i. It is the process in which the temperature of the system remains constant. The temperature change happens at such a slow rate that thermal equilibrium is maintained. For an isothermal process constant temperature , the ideal gas equation can be given as. That is, the pressure of the gas varies inversely with the volume of the gas. For an ideal gas, the internal energy depends only on the temperature. Thus, if the temperature is constant in an isothermal process, there is no change in internal energy. Adiabatic process is a type of thermodynamic process in which where there is no heat transfer. In other words, an adiabatic process occurs at constant heat. In an adiabatic process, the system is insulated from the surroundings and no heat is absorbed or released.
While putting the ice into the icebox, no heat will go out and no heat will come in. Thermodynamics uses the concepts of the isothermal process, isochoric process, isobaric process, and adiabatic process to explain the behaviour of a thermodynamic system and its relationship to temperature changes. Usually, when studying the chemical reactions, thermodynamically speaking, first, the effects under isothermal conditions are analyzed before moving on to analyze the temperature effect on the process.
As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Whereas in the isothermal process, the temperature remains constant throughout the work. This article will definitely help to understand the difference between isothermal and adiabatic process. In thermodynamics , we study the systems and objects in relation to the measurement of their temperatures, motions, and other physical characteristics. This is applicable to anything from the single-celled organisms to the mechanical systems in the universe.
Home » Physics » Class » What is the difference between isothermal and adiabatic process Class 11? As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Whereas in the isothermal process, the temperature remains constant throughout the work. An adiabatic process is one in which there is no supply of heat to the body undergoing change of thermodynamic state. In other words, the body is in adiabatic isolation. An isothermal process is a thermodynamic change where the temperature of the body does not change. An adiabatic process is defined as. The thermodynamic process in which there is no exchange of heat from the system to its surrounding neither during expansion nor during compression. Isothermal process is a process in which the temperature remains constant whereas adiabatic process is a process in which the heat of the body remains constant.
Difference between isothermal and adiabatic process class 11
We can now comprehend the difference between adiabatic and isothermal :. The behavior of a thermodynamic system and its relationship to temperature changes are described by the ideas of the isothermal process, isochoric process, isobaric process, and adiabatic process in thermodynamics. JEE Main. Topic Name:. Difference Between Isothermal and Adiabatic Process. Academic Session:.
Martin direct vent propane wall heater
This is therefore both isothermal as well as adiabatic. As there is an increase in the temperature of the system the pressure of the system tends to be more than the volume. This happens when the surrounding temperature is greater than the temperature of the system and there is no thermal equilibrium maintained. Transfer of heat occurs in this process. Relative Error Formula. Login To View Results. Hence it is known to be a reversible process. Two essential conditions for the adiabatic process are- The system should be completely insulated from the surrounding. However, when the temperature of the system decreases, the energy is released to the surroundings in the form of heat. It does not contain the transfer of heat. This is another type of phenomenon in thermodynamic, which is an adiabatic process. Thus, under certain conditions to specify a unique process, it is not sufficient to mention that the process is isothermal. Let us have some examples.
As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Whereas in the isothermal process, the temperature remains constant throughout the work.
Difference between Project Management and Process Management. Photoelectric Effect Experiment. Examples of the adiabatic process: There are many instances, some are as follows: In this process, gas compression is happening with the generation of heat. Thermodynamics uses the concepts of the isothermal process, isochoric process, isobaric process, and adiabatic process to explain the behaviour of a thermodynamic system and its relationship to temperature changes. In an adiabatic process, there is no transfer of any heat or mass between the system and the surroundings. Thus, for an isothermal process, the change in temperature will always be zero for an ideal system. Please Login to comment However, if the freezing occurs rapidly and there is no time for heat transfer, the process can be approximated as adiabatic. What is Adiabatic Process? Adiabatic Expansion. Joule-Thompson expansion and also the free expansion of ideal gases are the best example of such a system involving both processes at the same time. Suggest changes. The pressure is less at a given volume. Heat is the energy in transit, but the temperature is the property of a system like pressure and volume.

In it something is. Earlier I thought differently, thanks for the help in this question.
It is simply ridiculous.
I regret, that, I can help nothing, but it is assured, that to you will help to find the correct decision.