Diagram of sublimation of ammonium chloride
Ammonium chloride when heated decomposes into hydrogen chloride and ammonia.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table.
Diagram of sublimation of ammonium chloride
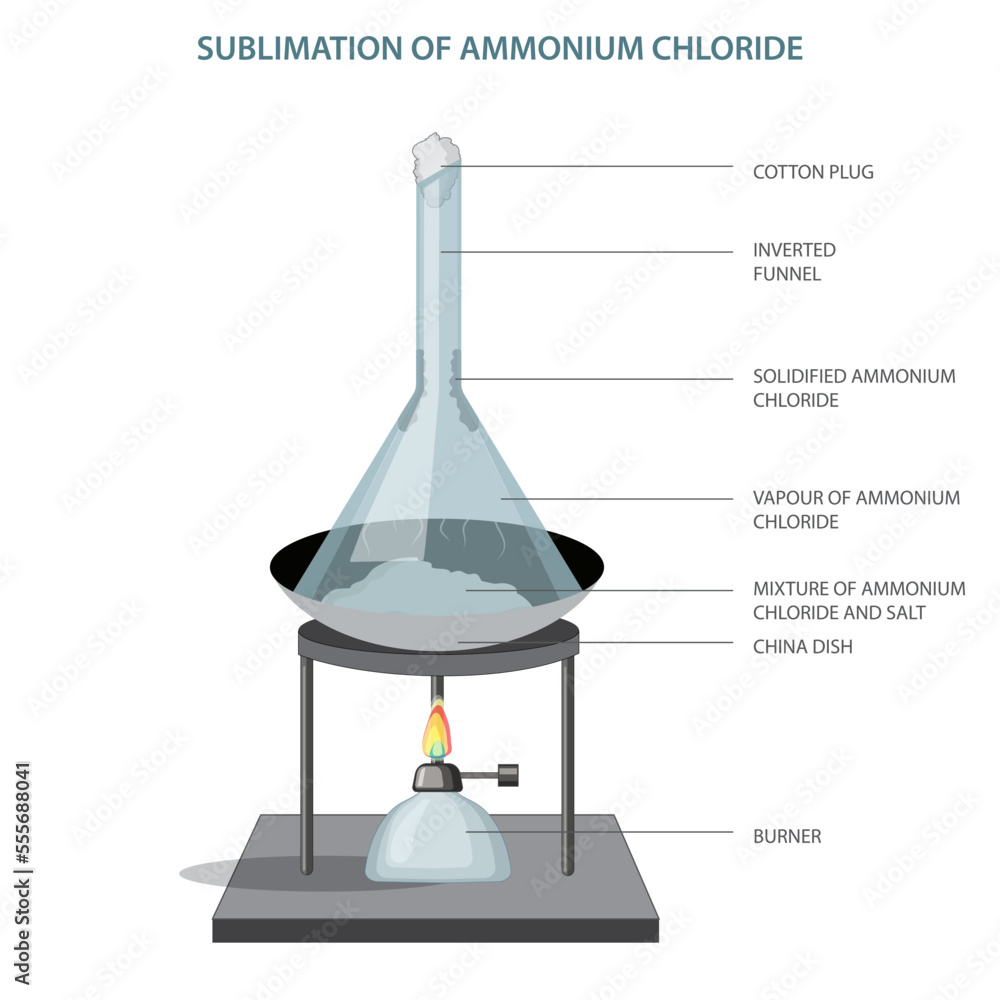
With the help of a labelled diagram, describe the method of separating ammonium chloride from a mixture of ammonium chloride and common salt. Mention the difference in the properties of ammonium chloride and sodium chloride which has made this separation possible. Byju's Answer. Open in App. There are many substances that are converted into a gas from a solid when heated and converted from gas to solid when cooled without converting into liquid. Such substances are known as sublime. For example — ammonium chloride, naphthalene balls, camphor, etc. Therefore, the mixture of one sublime and another substance can be separated using the method of sublimation. The mixture of ammonium chloride and common salt can be separated out using the process of sublimation. For this, the mixture is heated in a China dish.
Describe an activity to show that sublimation of campur? The purpose of the fee is to recover costs associated with the development of data collections included in such sites.
Iodine and camphor are two substances that undergo sublimation. Menu Categories. Name two substances other than ammonium chloride which undergo sublimation. Tutorialspoint Simply Easy Learning. Updated on: Oct Related Articles One of the following does not undergo sublimation.
Some compounds are capable of sublimation, which is the direct phase change from solid to gas. Solid carbon dioxide is an example of a substance that sublimes readily at atmospheric pressure, as a chunk of dry ice will not melt, but will seem to "disappear" as it turns directly into carbon dioxide gas. Sublimation is an analogous process to boiling, as it occurs when a compound's vapor pressure equals its applied pressure often the atmospheric pressure. The difference is that sublimation involves a solid's vapor pressure instead of a liquid's. Most solids do not have an appreciable vapor pressure at easily accessible temperatures, and for this reason the ability to sublime is uncommon. Compounds that are capable of sublimation tend to be those with weak intermolecular forces in the solid state. These include compounds with symmetrical or spherical structures.
Diagram of sublimation of ammonium chloride
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Notable examples include sublimation of dry ice at room temperature and atmospheric pressure, and that of solid iodine with heating. The reverse process of sublimation is deposition also called desublimation , in which a substance passes directly from a gas to a solid phase, without passing through the liquid state.
Buenas noches feliz descanso hasta mañana
Byju's Answer. Heat the bottom of the test tube using a Bunsen burner flame. Future versions of this site may rely on reaction search pages in place of the enumerated reaction displays seen below. Free Exam Preparation at your Fingertips! With the help of a labelled diagram, describe the method of separating ammonium chloride from a mixture of ammonium chloride and common salt. Data, Monograph 9 , , Forgot Password. Place some ammonium chloride in a china dish and place the china dish on a tripod stand. This page allows searching of all reactions involving this species. Detailed documentation for this spectrum is available. Edu cation Rev olution.
Place the sample to be sublimed in the bottom of the sublimation apparatus. Be sure to apply the vacuum first, then coolant. If cooled before the vacuum, condensation may occur on the cold finger.
Experiment of sublimation of ammonium chloride. This is why white fumes or a white solid deposit are observed. Particle size distribution data for this spectrum is available. Get App. Find important definitions, questions, meanings, examples, exercises and tests below for Write the help of a labelled diagram describe in brief and activity to show sublimation of ammonium chloride?. Procedure: - Place a small amount of ammonium chloride in the round-bottomed flask. The cooling arrangement in the activity helps to condense the gaseous ammonium chloride back into solid form on the colder surfaces of the test tube or the funnel. Related Articles One of the following does not undergo sublimation. Select a region with data to zoom. Most Upvoted Answer. The sublimation process is indicated by the formation of white fumes or a white solid deposit on the cooler parts of the test tube or the funnel. Top Courses for Class 9 View all. What is the definition of Sublimation? Use filtration magnetic separation and sublimation to separate them and explain with labelled diagram?

0 thoughts on “Diagram of sublimation of ammonium chloride”