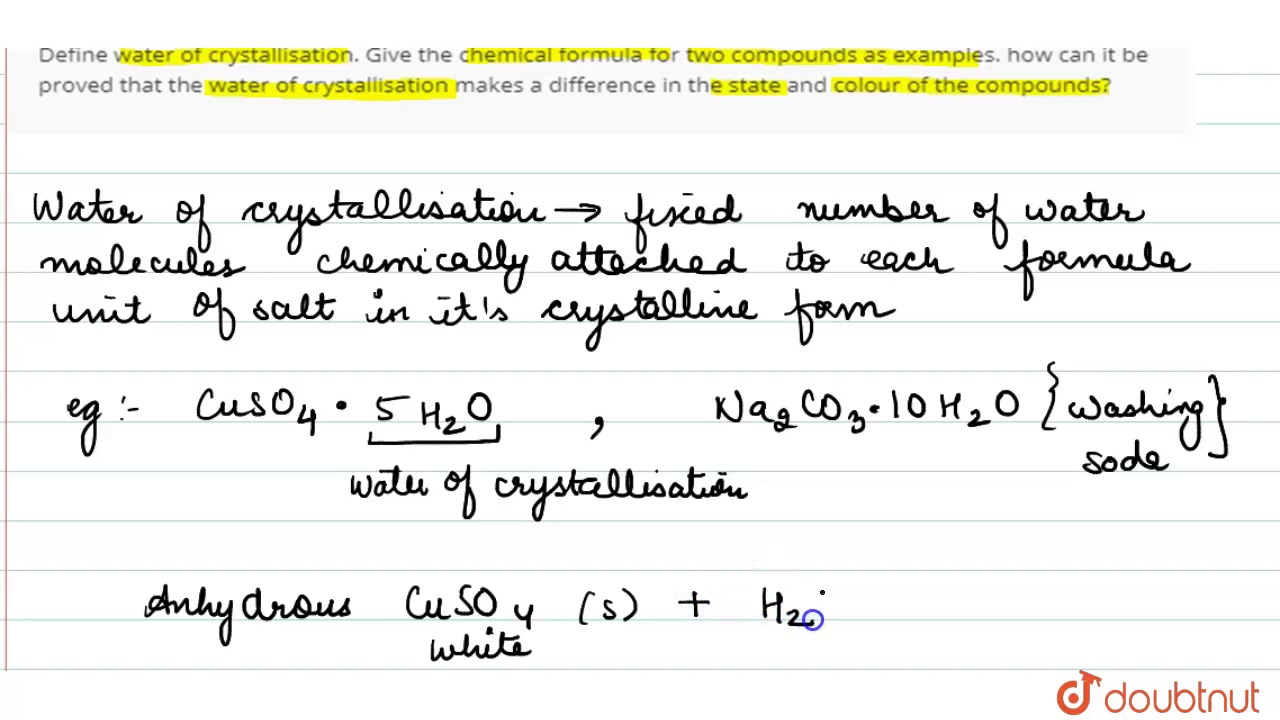
Define water of crystallization class 10
Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. This is the most frequent method for purifying seawater.
Last updated at May 29, by Teachoo. Copper Sulphate CuSO 4 is ideally white and powdery and does not have any water anhydrous. When we add water to these crystals they turn blue due to the formation of Copper pentahydrate CuSO 4. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Your browser does not support the audio element. Maths Classes.
Define water of crystallization class 10
The chemical formulae of two compounds can be same. Do you agree? Water of crystallisation in compound P:. What is meant by water of crystallisation in a substance? How would you show that blue copper sulphate crystals contain water of crystallisation? How many water molecules are present as water of crystallisation in gypsum? What is water of crystallisation? Write the common name and chemical formula of a commercially important compound which has ten water molecules as water crystallisation. How is this compound obtained? Write the chemical equation also. List any two uses of this compound. How many molecules of water of crystallisation are present in blue vitriol? How mariy molecules of water of crystallisation are present in blue vitriol?
Facebook Whatsapp. But all solvents can be found in some host crystals. Acta Crystallogr.
In chemistry, water s of crystallization or water s of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt , which is not directly bonded to the metal cation. Upon crystallization from water, or water-containing solvents , many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to inorganic salts , proteins crystallize with large amounts of water in the crystal lattice.
Question 1 What is meant by water of crystallisation? Give example? Question 2 What are hydrated salts? Question 3 What are anhydrous salts? Question 4 Name a compound which contains two molecules of water of crystallisation? Question 5 Why copper sulphate is blue in colour?
Define water of crystallization class 10
In chemistry, water s of crystallization or water s of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt , which is not directly bonded to the metal cation. Upon crystallization from water, or water-containing solvents , many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to inorganic salts , proteins crystallize with large amounts of water in the crystal lattice. Knowledge of hydration is essential for calculating the masses for many compounds. The reactivity of many salt-like solids is sensitive to the presence of water.
The lion guard season 3
Compared to inorganic salts , proteins crystallize with large amounts of water in the crystal lattice. Work Experiences. Article Talk. When hydrated salts are heated to high temperatures, they lose their crystallisation water. European Journal of Inorganic Chemistry. Solve all your doubts with Teachoo Black! These water molecules are referred to as crystallisation fluids or hydration waters. Carbon, a member of group 14 forms a large number of carbon compounds Campus Experiences. How will use two identical glass prisms so that a narrow beam of white View Text Solution. The water of crystallization is a part of the crystal structure of water. Contents move to sidebar hide. Please go through our recently updated Improvement Guidelines before submitting any improvements. The hydration and dehydration of salts is central to the use of phase-change materials for energy storage.
Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. This is the most frequent method for purifying seawater.
Report issue Report. Categories : Crystallography Hydrates. Question 6: Write the name and formula of a salt that has five molecules of crystallisation water in it. The water molecules in a hydrated salt are incorporated into the crystalline structure of the salt. Old search 2. What is Water Pollution? Knowledge of hydration is essential for calculating the masses for many compounds. What happens when food materials containing fats and oils are left for Lead nitrate solution is added to a tes tube containing potassium iodi Question 1: Which of the following salts has no Water of Crystallization?

Probably, I am mistaken.
Should you tell it � a lie.