Daniell cell class 12
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathoderespectively. Both metals daniell cell class 12 submerged in the corresponding salt solutions.
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments. I would like to thank my parents for supporting my work on this project. One of their oldest and most simple incarnation was the Daniell cell.
Daniell cell class 12
How does a Cell in a T. V remote make it work or how a Battery of Mobile Phone Charges when connected to its charger? All such questions are answered in the branch of Science known as Electrochemistry. Electrochemistry is the study of producing Electricity through Chemical reactions and also the use of Electricity to carry out non-spontaneous Chemical reactions. To achieve the above-mentioned aim Cells are used. Cells are devices in which Chemical Reactions due to Electricity or produces Electricity. ElectroChemical Cells. Electrolytic Cells. These Cells are those Cells that produce Electricity through Chemical reactions. Chemical Energy is converted into Electrical Energy by the Cells. These Cells are those Cells which use Electricity to carry out non-spontaneous Chemical reactions. The basic difference between ElectroChemical Cells and Electrolytic Cells are listed in the table below:. Electrolytic Cell. The anode is negative, the cathode is positive.
What is the main function of a salt bridge? At the cathode positive electrodecopper is reduced as per the following reaction:. Search inside document.
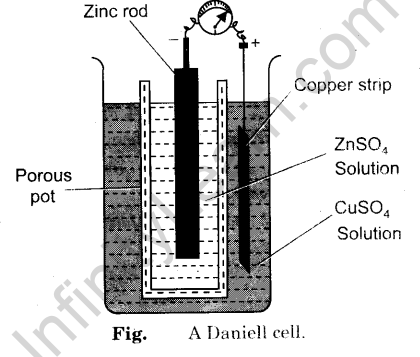
In this article, you will learn in detail about Daniell cell, its definition, construction, the chemical reaction involved, and its applications. Read on for more. A Daniell cell is a type of electrochemical cell that consists of a copper pot that is filled with a solution of copper II sulphate. An unglazed earthenware is immersed in this solution containing sulphuric acid and a zinc electrode. The Daniell cell was invented, while the chemist was seeking a way to eliminate the hydrogen bubble issue found in the voltaic pile.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell. The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate. Register Now. A conventional galvanic cell, it is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions. A copper vessel makes up this cell. In this case, a saturated CuSO 4 solution is used as a depolarizer and diluent. Fill with H 2 SO 4 , which works as an electrolyte. Zn 2 SO 4 is used to submerge a zinc rod that has been amalgamated. CuSO 4 crystals are kept in touch with CuSO 4 solution by a transparent layer just below the upper surface of copper vessels, ensuring that the solution is always saturated.
Daniell cell class 12
A Daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulphate and copper sulphate respectively. A typical galvanic cell, it is designed to make use of the spontaneous redox reaction between zinc and cupric ion to produce an electric current. This cell consists of a copper vessel. In which saturated CuSO 4 solution is filled which acts as depolarizer and dil. H 2 SO 4 is filled which acts as an electrolyte. An amalgamated zinc rod is immersed in Zn 2 SO 4.
Daphne hot
We receieved your request. School Board Live Online classes. Battery refers to a group of Cells combined. They are completed by the salt bridge. Here the chemical potential energy is converted to thermal energy which is indicated by the warmth of the reaction vessel. A disadvantage of the gravity cell is that a current has to be continually drawn to keep the two solutions from mixing by diffusion, so it is unsuitable for intermittent use. Here, a circuit external to the device allows current to move from a copper electrode to a zinc electrode or a cathode to an anode. A galvanic cell converts chemical energy into electrical energy, while an electrolytic cell converts the reverse. Nevertheless, the Daniell cell provides a longer and more reliable current than the Voltaic pile because the electrolyte deposited copper, which is a conductor, rather than hydrogen, which is an insulator, on the cathode. This is due to reduction in Chemical energy. Salt bridges balance charges.
How does a Cell in a T.
Electrochemical Cell Electrochemical Cell. A copper disc perforated with numerous holes was placed across the cylinder recessed down from the top. Review and Revise Your Notes at Home. The salt bridge completes the circuit. Please Enter valid email. Chem Project. JEE Application Process. Volatic or Galvanic Cell. This top layer is kept separate from the bottom copper sulfate layer by its lower density and by the polarity of the cell. One can find its uses in electrometallurgy and electrotyping. Wikimedia Commons has media related to Daniell cell. Change number Resend OTP. Experiment In the Daniell cell, copper and zinc electrodes are immersed in a solution of copper II sulfate and zinc sulfate, respectively. These Cells are those Cells that produce Electricity through Chemical reactions. This explanation is very good and understandable.

I apologise, but, in my opinion, you commit an error. Let's discuss it.
I apologise, but, in my opinion, you commit an error. I can defend the position.