Correct order of energy of 2s orbital
Submitted by Charles N.
According to aufbau principle, the correct order of energy of 3d,4s and 4p-orbitals is. The correct order of energies of d-orbitals of metal ion in a square planar complex is. The correct order of energies of d-orbitals of metal ion in a square planar complex is :. The correct orders of increasing energy of atomic orbitals is. The correct order of increasing energy of atomic orbitals is. The correct order of electropositive nature of Li,Na and K is. Which is the correct order of increasing energy of the listed orbitals in the atom of titanium?
Correct order of energy of 2s orbital
.
The energy of an electron in an atom is related to its distance from the nucleus.
.
The energy of an electron in a single atom can be determined solely by the principal quantum number. However, the energy of an electron in multi-electron atoms depends on both its principal quantum number n and its azimuthal quantum number l. This difference in energy of various subshells residing in the same shell is mainly attributed to the mutual repulsion among the electrons in a multi-electron atom. In multi-electron atoms, there is a repulsive force acting between various electrons apart from the attractive force between the nucleus and the electrons. Thus, the stability of an electron in a multi-electron atom is dependent on the total attractive interactions and the repulsive interactions. The electron in an atom is only stable if the total attractive interaction is more than the total repulsive interaction. For bigger atoms, due to the presence of electrons in the inner shells, the electrons in the outer shell are deprived to experience the full positive charge of the nucleus Z e. This effect is known as the shielding of the outer shell electrons from the nucleus by the inner shell electrons. The net positive charge experienced by the outer shell electrons is termed as the effective nuclear charge Zeff.
Correct order of energy of 2s orbital
Scientists needed a new approach that took the wave behavior of the electron into account. It successfully describes the energies and spatial distributions of electrons in atoms and molecules. He was notorious for his intense dislike of memorizing data and learning from books.
Square pop ceiling design
The number of isomers which are ethers and having the molecular formul The role of haemoglobin is to. Ammonia is NOT produced in the reaction of :. Suggested Textbook. Try Numerade free for 7 days View This Answer. Sign Up. The correct order of energies of d-orbitals of metal ion in a square planar complex is. Reviewed By Expert Numerade Educators. Log in to watch this video The correct orders of increasing energy of atomic orbitals is. Chemistry
An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Electrons, however, are not simply floating within the atom; instead, they are fixed within electronic orbitals.
The chlorine atom of the following compound that reacts most re The correct order of electropositive nature of Li,Na and K is. The number of C - C sigma bonds in the compound. Already have an account? Which is the correct order of increasing energy of the listed orbitals in the atom of titanium? The numbers of lone pair and bond pairs in hydrazine are, respectively Ask unlimited questions and get video answers from our expert STEM educators. More Than Just We take learning seriously. The hybridisation of xenon atom XeF 4 is Try Numerade free for 7 days View This Answer. Upgrade to add a comment. Which order of energies of orbitals is correct in a many electron atom? Get Better Grades Now.

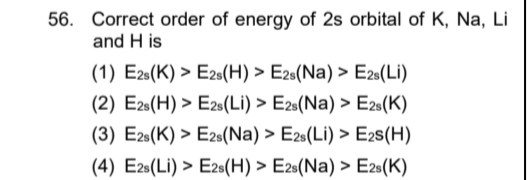
Here and so too happens:)
It is scandal!
I consider, that you are not right. Let's discuss.