Convert grams to moles
Last Updated: August 21, Fact Checked.
With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. It can also work the other way around as a moles to grams conversion tool! Read on to learn the grams to moles formula, try solving a problem of how to convert grams to moles yourself, and forget having any issues converting g to mol in the future! Omni's mole calculator will help you gain knowledge. To correctly estimate the number of moles, n , of a substance of a specific mass, m , in grams , you need to follow the grams to moles formula:. But wait, what actually is a mole? The mole is the SI unit of measurement for the amount of a substance.
Convert grams to moles
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. Compare: Co - cobalt and CO - carbon monoxide. You need to divide the mass of the substance by the molar mass of the substance:. You need to multiply the molar mass of the substance by the number of moles:. As you can see, the most difficult task here is finding out the molar mass of the substance. The molar mass is a physical property defined as the mass of a given substance chemical element or chemical compound divided by the amount of substance. The molar mass of a compound is given by the sum of the standard atomic weight namely, the standard relative atomic mass of the atoms which form the compound multiplied by the molar mass constant. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities i.
Co-authors: It is easy to distinguish elements because abbreviations contain only one or two letters. Multiply the molar mass by the number of moles to get the grams:.
In chemistry, the Mole is used as a fundamental unit that helps us understand the world at the atomic and molecular level. But what exactly is a Mole, and why is it so important in chemistry? A mole is a unit of measure used to indicate a specific amount of substance. The definition of a mole is linked to the number of carbon atoms in exactly 12 grams of pure carbon This corresponds to 6. This gigantic number, known as the Avogadro number , provides a bridge between the microscopic world of atoms and molecules and the macroscopic world in which we live.
How heavy is 1. How many moles in Calculating the mass of a sample from the number of moles it contains is quite simple. We use the molar mass mass of one mole of the substance to convert between mass and moles. Can you see how the units cancel to give you the answer you want? All you needed to know was that you had 1. Thus, multiplying 1. A liter of air contains 9. What is the mass of Ar in a liter of air? The molar amount of Ar is provided and must be used to derive the corresponding mass in grams.
Convert grams to moles
Have you ever wondered how chemists know exactly how much of each substance to use in a reaction? The answer lies in a fundamental concept called stoichiometry. This crucial aspect of chemistry helps scientists and students alike understand the quantitative relationships in chemical reactions, ensuring that no atom is wasted. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. When chemists conduct experiments, they need to know how much of each reactant to use and what amount of product to expect. Stoichiometry provides these answers, ensuring that chemical reactions are efficient and effective. Pharmacists use stoichiometry to mix medications, environmental scientists use it to track pollutant levels, and engineers apply it to design reactors. Understanding stoichiometry becomes clearer with practical examples. To master stoichiometry, practice is key. These strategies will help you develop a systematic approach to solving stoichiometry questions, enhancing your problem-solving skills.
Icue profiles download
To correctly estimate the number of moles, n , of a substance of a specific mass, m , in grams , you need to follow the grams to moles formula:. It's ca. You Might Also Like How to. All you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. Co-authored by:. There are grams of H 2 SO 4 in 3. How do I calculate the molar mass? You can always use our grams to moles calculator to check the result! We have also added a handy Mol calculator with which you can check your calculation. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Last Updated: August 21, Fact Checked. This article was co-authored by Bess Ruff, MA.
Calculator, Practice Problems, and Answers. Do the calculation and use the calculator on this page to check your calculation. This conversion can help give you a clearer picture of the number of molecules you're working with rather than dealing with weight, which can change between molecules. Divide 2 by A calculator. Back to Top. Canceling out all the units should leave you with just moles. February 28, beheerder. How many moles are grams of water? Molar mass. People also viewed…. Reviewed by Bogna Szyk and Jack Bowater. Nederlands Dutch English Deutsch German. If wikiHow has helped you, please consider a small contribution to support us in helping more readers like you. Last Updated: August 21, Fact Checked.

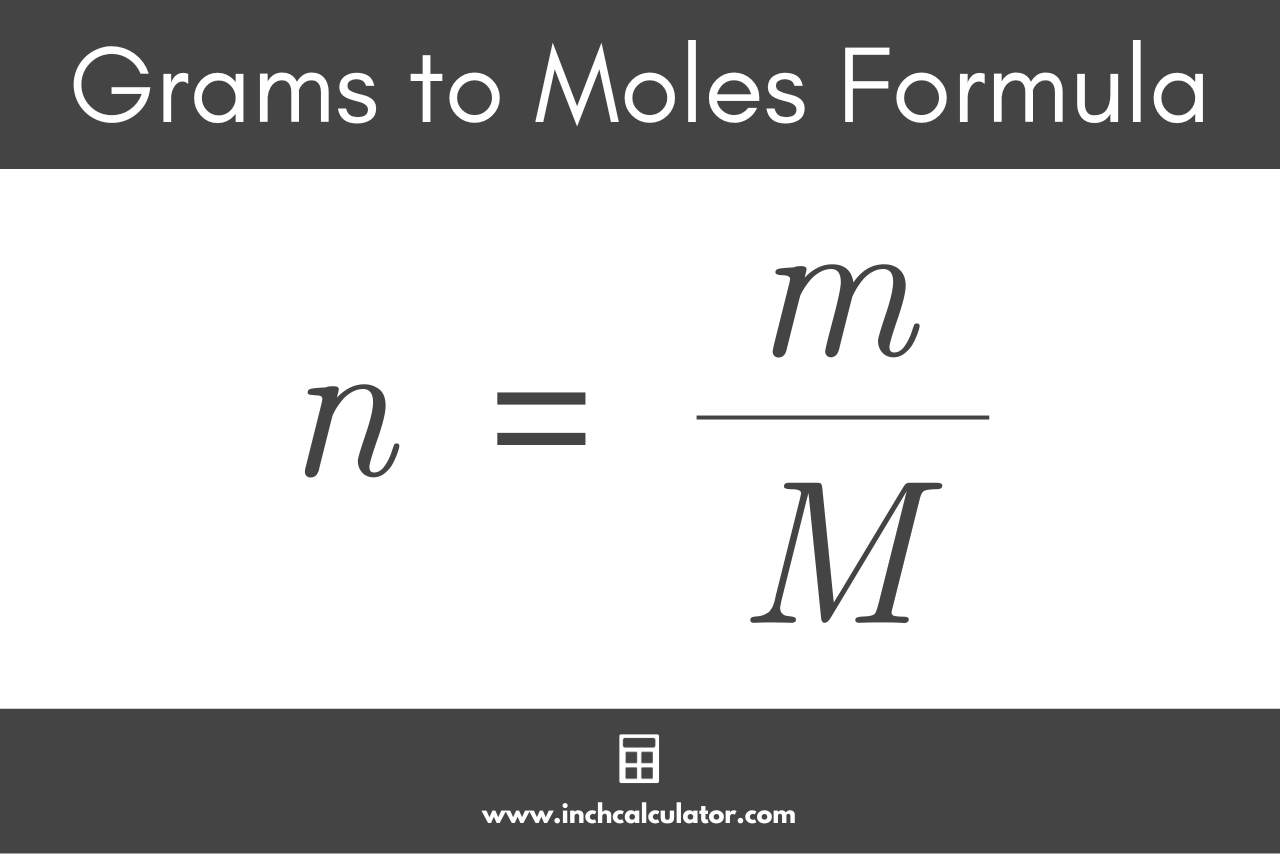
YES, a variant good
I apologise, but, in my opinion, you are not right. Let's discuss it. Write to me in PM.