Cl2 lewis
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group.
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Cl2 lewis
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell. By dividing the total valence electrons by two, we find the total electron pairs. For the Cl 2 molecule, there are seven electron pairs in their valence shells. To get the best Lewis structure, reduce charges on atoms by converting lone pairs to bonds. As there are no charges on atoms, there is no need to reduce charges. We already have the best Lewis structure for Cl 2. As the overall formal charge is zero, the above Lewis structure of Cl 2 is the most appropriate and stable. Cl 2 has a linear electron geometry.
FREE Signup. Read more about our Editorial process.
Chlorine molecule consists of two chlorine atoms. At room temperature, it is a yellow gas with a pungent odor. It has a high density 3. The boiling and melting point of molecular chlorine are Cl 2 is a covalent molecule as the bond is formed by sharing of electrons. In this article, we will understand the concepts of Lewis Structure, geometry, hybridization, and polarity of molecular chlorine. Lewis Structure is a simple depiction of valence shell electrons in a molecule.
Transcript: OK, we're going to draw the dot structure for Chlorine gas—a poisonous green gas. This is Dr. And we'll start out by figuring out how many valence electrons we have for Chlorine. On the periodic table, it's in group 7 or 17, so it has 7 valence electrons; but we have two of them, so let's multiply that times two for a total of 14 valence electrons. So we're going to spread those around the atoms, fill the octets, form a chemical bond. So here's Chlorine, and another Chlorine. Let's start by putting two valence electrons between the Chlorines.
Cl2 lewis
Struktur Cl2 Lewis memiliki dua atom klor Cl yang mengandung ikatan tunggal di antara keduanya. Ada 3 pasangan elektron bebas pada dua atom klor Cl. Jika Anda tidak memahami apa pun dari gambar struktur Lewis Cl2 klorin di atas, ikuti terus saya dan Anda akan mendapatkan penjelasan rinci langkah demi langkah dalam menggambar struktur Lewis Cl2. Jadi mari kita lanjutkan ke langkah-langkah menggambar struktur Lewis Cl2. Untuk mengetahui jumlah total elektron valensi dalam molekul Cl2 klorin , pertama-tama Anda perlu mengetahui elektron valensi yang ada dalam satu atom klor. Elektron valensi adalah elektron yang ada di orbit terluar atom mana pun. Di sini saya akan memberi tahu Anda cara mudah mencari elektron valensi klorin menggunakan tabel periodik.
How to round in c++
We consider the geometry and the shape of Cl 2 to be linear. Now, you have come to the final step and here you have to check the formal charge on the chlorine atoms Cl. This indicates that both the chlorine Cl atoms are chemically bonded with each other in a Cl2 molecule. The boiling and melting point of molecular chlorine are More Articles for Chemistry. By doing so, you will get the following lewis structure of Cl2. One bond exists between two chlorine atoms in the Cl2 Lewis structure. Share Share Share Call Us. Structure, Synthesis, and Reactions - Testbook. Preparation of Chlorine gas.
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group.
Interstitial Compounds. Here, I have explained 6 simple steps to draw the lewis dot structure of Cl2 along with images. That means they have 8 electrons. So the left side chlorine is the outer atom. For Cl 2 , there is one side atom. Count the number of valence shell electrons on the central atom and let it be equal to A arbitrary variable. The polarity of a compound depends on the presence or absence of net dipole moment. Test Series. Want to know more about this Super Coaching? Valence electrons are the electrons that are present in the outermost orbit of any atom. Valence electrons are the number of electrons present in the outermost shell of an atom. The geometry and bonding of some polyatomic covalent compounds are explained using a unique concept called hybridization. This step can be avoided for neutral compounds. Leave a Comment Cancel Reply Your email address will not be published.

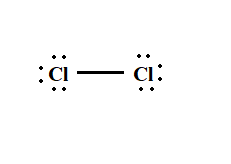
0 thoughts on “Cl2 lewis”