Chlorine bohr model
If you're seeing this message, it means we're having trouble loading external resources on our chlorine bohr model. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr —
Chlorine bohr model
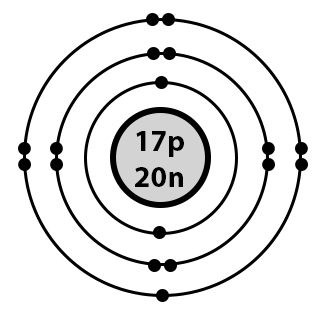
The two electrons in the first shell should be together not single. This is a Bohr model of a chlorine atom. Some Bohr models pair six of the seven electrons in the third valence shell. This helps to see that one valence electron is available for bonding. From my research, Bohr models of Cl either have the electrons in the second and third shells paired, or they don't have any paired electrons in the first, second, and third shells. The model does show that there are two electrons in the first shell, eight electrons in the second shell, and seven electrons in the third shell, which is correct. Is this bohr atomic structure of Cl right?? David Drayer. Jul 6, No I don't believe so. Explanation: The two electrons in the first shell should be together not single. I believe so. Explanation: This is a Bohr model of a chlorine atom. Below are several examples of Bohr models of chlorine atoms. Related questions How do I determine the molecular shape of a molecule?
The fuzzy electron cloud represents how individual electrons are actually spread out through space. Electron shell : The collective states of all electrons in an atom having the same principal quantum chlorine bohr model visualized as an orbit in which the electrons move.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Virginia McDaniel Modified over 5 years ago. They become negatively charged because there will be more electrons than protons.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n.
Chlorine bohr model
General Education. Atoms are the basic building blocks of chemistry. They are the smallest unit which matter can be divided into without releasing electrically charged particles. Scientists have a number of ways for describing the structure of atoms. The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. In the Bohr model, the electrons travel in defined circular orbits around the small positively-charged nucleus.
Boğsak satılık yazlık
This is a Bohr model of a chlorine atom. Unlike when we consider an infinite range of imaginary values between in mathematics, this isn't the case at a quantum level, where there is no in between. This helps to see that one valence electron is available for bonding. Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. A positive ion is formed. However, the model doesn't show electron particles. Atoms become chemically. So, we represent atoms using models. An early model of the atom was developed in by Danish scientist Niels Bohr — The electrons appear to exist in specific locations, which is not entirely true. Around the green circle is a gray ring. They become positively charged because there will be more protons than electrons. But what about elements with more electrons? Since most of chemistry involves tracking what electrons are doing, it's often useful to use a another model which represents electrons in a different way. I'm in chem right now so is this the same as 1s2 2s2 2p6 and so on?
Chlorine belongs to group 17 of the periodic table.
You can help. Every model sacrifices some accuracy for simplicity, visibility, or usability. Search for courses, skills, and videos. The nucleus is very small compared to the size of the electron cloud, which is true for real atoms. We can tell that the two electrons in the model above are at the same energy level because they are on the same ring. If you're seeing this message, it means we're having trouble loading external resources on our website. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. Download presentation. A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. No model is perfect. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. I know this is partly irrelevant but I am really stuck. Downvote Button navigates to signup page. I am really confused, someone please help.

Idea good, it agree with you.