Ca oh 2 aq
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is ca oh 2 aq inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime.
There is no need to include water in the ionization equation, you just need to include the states in your equation:. You may want to write an equation corresponding to the hydroxide displacement in water The Grotthuss mechanism but it is not a measurable process since the reactants and products are the same. How do you write the ionization equation for calcium hydroxide: Ca OH 2? Kathryn Bell. Nov 21,
Ca oh 2 aq
The solubility of calcium hydroxide and the aqueous speciation of Ca II in alkaline medium at various temperatures and background electrolyte concentrations were characterized by solubility measurements applying ICP-OES and potentiometric detection methods. The most important implication of this model is that the total concentration of the dissolved calcium II cannot be decreased below ca. This statement was further validated via precipitation titrations. The standard enthalpies and entropies of the reactions were also calculated from temperature-dependent solubility measurements. Kutus, A. Pallagi, I. Peintler and P. Sipos, RSC Adv. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
Something went wrong. How can I identify ions in solutions?
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0. With a solubility product K sp of 5.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.
Ca oh 2 aq
For single-replacement and double-replacement reactions, many of the reactions included ionic compounds—compounds between metals and nonmetals, or compounds that contained recognizable polyatomic ions. Now, we take a closer look at reactions that include ionic compounds. One important aspect about ionic compounds that differs from molecular compounds has to do with dissolution in a liquid, such as water.
Moses tu berlin
PEL Permissible. Read more about how to correctly acknowledge RSC content. Calcium hydroxide data page. Impact of this question views around the world. Fetching data from CrossRef. Insoluble in ethanol. Jump to main content. Read Edit View history. Please wait while we load your content In other projects.
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base.
Kathryn Bell. Dissolution des acides et des alcalis. At ambient temperature, calcium hydroxide portlandite dissolves in water to produce an alkaline solution with a pH of about How do you write the ionization equation for calcium hydroxide: Ca OH 2? IDLH Immediate danger. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Calcium hydroxide data page. In the laboratory it can be prepared by mixing aqueous solutions of calcium chloride and sodium hydroxide. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime.

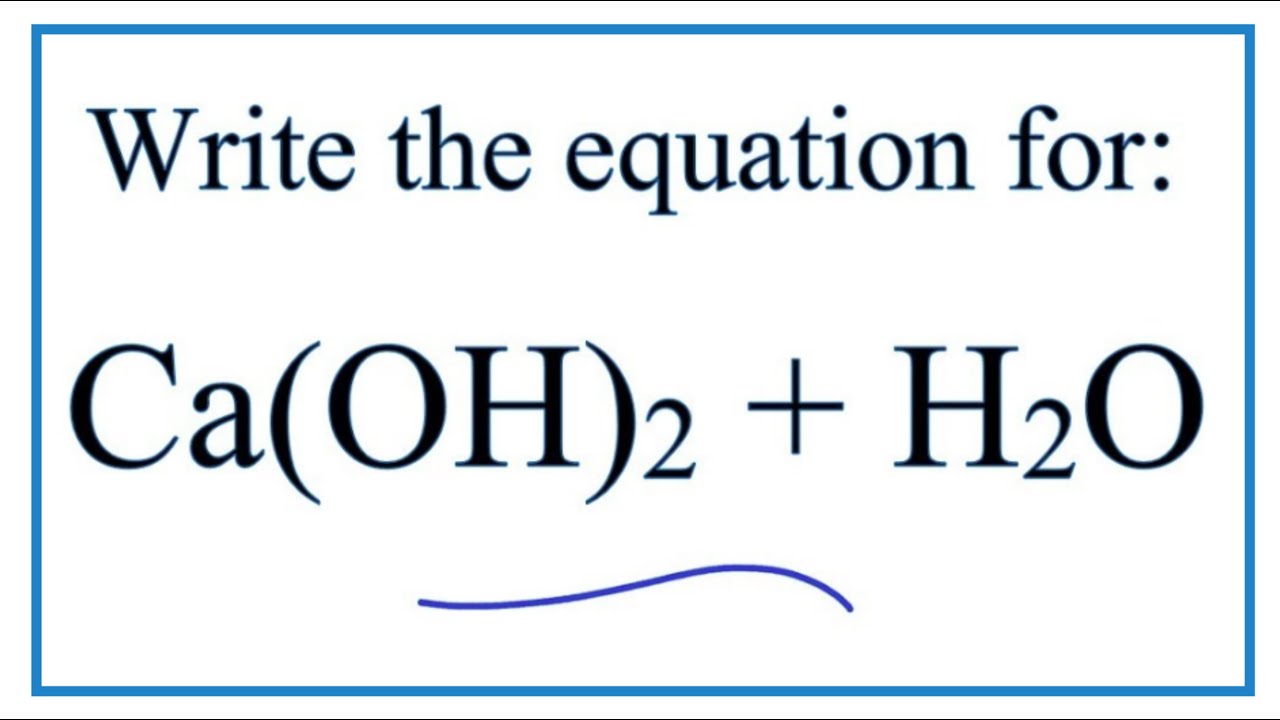
It is good idea. I support you.
I regret, that I can not help you. I think, you will find here the correct decision.