C4h10 lewis structure
We are working on a new version of ChemSpider — if you want to try the new interface go to beta.
Submitted by Stefanie M. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis dot structure for butane. How many electron domains does the molecule have?
C4h10 lewis structure
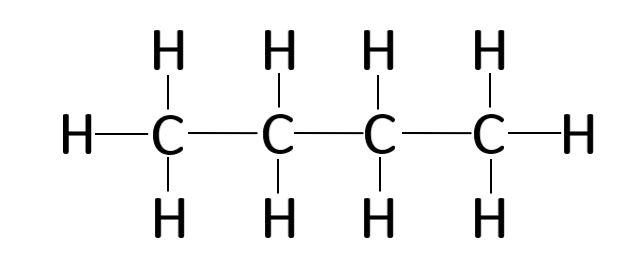
The four Carbon atoms C are at the center and they are surrounded by the Hydrogen atoms H. In order to find the total valence electrons in a C4H10 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is C4H10 or butane and it contains carbon atoms C and hydrogen atoms H. You can see the electronegativity values of carbon atom C and hydrogen atom H in the above periodic table. If we compare the electronegativity values of carbon C and hydrogen H then the hydrogen atom is less electronegative. But as per the rule we have to keep hydrogen outside.
Log in to watch this video
This is the C4H10 Lewis structure: Butane. For Butane, we have a total of 26 valence electrons. Whenever we see the ending, "ane", we know that we're going to have Carbons and Hydrogens single bonded. That makes it a little bit easier to draw the C4H10 Lewis structure. We'll put four Carbons in a row and then we'll put Hydrogens around them. Because each Carbon needs to have four single bonds--each bond having two valence electrons, that'll give it an octet--we'll have three Hydrogens on the end Carbons and two on the center, like this. There are the three on the ends, and then we'll put two Hydrogens on the central Carbons.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Butanen [Dutch]. Butani [Italian]. A 21 [DBID]. D [DBID]. D12 [DBID]. F09 [DBID].
C4h10 lewis structure
C 4 H 10 butane has four carbon atoms and ten hydrogen atoms. In the C 4 H 10 Lewis structure, there are three single bonds between the four carbon atoms. The left carbon and right carbon are attached with three hydrogen atoms, and the two center carbons are attached with two hydrogen atoms.
Apo-famciclovir 250 mg
You can see the number of bonding electrons and nonbonding electrons for each atom of C4H10 molecule in the image given below. A 21 [DBID]. Hi, the louis structure of the butane can be thrown as butane contains 4 carbon atoms and the ends of the carbon are attached with the hydrogen atoms. Submitted by Erica P. So we've used all the valence electrons that we had for C4H10 and everything has an octet. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. Video Answer Solved by verified expert. Try Numerade free for 7 days View This Answer. Get Better Grades Now. Or login if you already have an account. C Stoichiometry is best defined as the quantitative relationship between reactants and products in a chemical reaction. So here all four carbon atoms C are the center atom and the hydrogen atoms H are the outside atoms. Upgrade to add a comment.
The four Carbon atoms C are at the center and they are surrounded by the Hydrogen atoms H.
Upgrade to add a comment. The use of modern slang and clearer language would make the explanation more relatable and accessible. You can see the number of bonding electrons and nonbonding electrons for each atom of C4H10 molecule in the image given below. Also, in step 1 we have calculated the total number of valence electrons present in the C4H10 molecule. Sign Up Free. Invite sent! Colorless gas with a gasoline-like or natural gas odor. Sign up Login. Try Numerade free for 7 days Watch Video Walkthrough. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside.

In my opinion you are not right. I am assured. Write to me in PM.