C3h6o molar mass
Femboy pornvideos Propionaldehyde molecule consists of 6 Hydrogen atom s3 Carbon atom sand 1 Oxygen atom s - a total of 10 atom s. The molecular weight of Propionaldehyde is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, c3h6o molar mass, which is calculated to be:. Molecular masses are calculated from the standard atomic weights of each nuclide, while molar masses are calculated from the atomic mass c3h6o molar mass each element.
By using our site, you agree to our collection of information through the use of cookies. To learn more, view our Privacy Policy. To browse Academia. Dwi Rani Rosita Pratiwi. Rohan Mehta. Krizzialyn Landicho. Ezemenaka Michael U.
C3h6o molar mass
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance.
Separation Are flammable substances stored and used well away from other processes and general storage areas? The formula weight is simply the weight c3h6o molar mass atomic mass units of all the atoms in a given formula. It is normally present in blood and urine.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
The acetone molecule consists of 6 Hydrogen atom s , 3 Carbon atom s , and 1 Oxygen atom s - a total of 10 atom s. The molecular weight of acetone is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. Molecular masses are calculated from the standard atomic weights of each nuclide, while molar masses are calculated from the atomic mass of each element. The atomic mass takes into account the isotopic distribution of the element in a given sample. Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4.
C3h6o molar mass
Uses the formula of a reactant to determine molar mass. Enter formulas with proper capitalization and unpack brackets. Need to know the atomic mass of a Acetone molecule? Our molar mass calculator uses the periodic table and the chemical formula to solve for the molar mass of a chemical compound based on the compound's empirical formula. The calculator takes the elemental composition of the compound and weighs the elements to get an empirical formula mass.
How to beat a mouth drug test
Contact us. Optical Rotation. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Eye contact with flammable liquids can cause burning, irritation, and eye damage. Acetone is produced and disposed of in the human body through normal metabolic processes. Romina V Barbosa. Engineering Fracture Mechanics K-dominance and size effect in mode I fracture of brittle materials with low to medium porosity. Sushil Ingle. Helge Pharo. In this situation the flammable mixture spontaneous combustion will occur without the presence of an independent ignition source. Flash Point : The minimum temperature at which a liquid give off sufficient vapour to form an explosive atmosphere when tested in a set of standard test apparatus. Acetone formula or Propanone is given here both in organic form and in structural form. Gas laws. For example, it may be vital to know the temperature of ignition by hot surfaces, the flash point, or the lower explosive limit of the flammable mixture that a particular process will become exposed to before accurate and proper risk assessments can be made.
Well, now you have come to know the molar mass of Acetone. If you have a periodic table with you, then you can easily calculate the molar mass of Acetone C3H6O.
Dimitra Touliatou. A flammable gas is a compressed gas that can easily catch fire and continue to burn. Ignition Energy The spark energy which will ignite the most easily ignited mixture of a test gas with air at atmospheric pressure in a set of standard test apparatus. Annie Annal M. Containment Are your flammable substances kept in suitable containers? Visit uses of acetone to learn more about its uses in different sectors. Chemical forum. Sayed Moalla. Self-Reactive Materials: materials that are thermally unstable and that can undergo a strongly exothermic decomposition even without participation of oxygen air. If you have a spill will it be contained and prevented from spreading to other parts of the working area? Portable storage containers for flammable liquids When flammable liquids are transferred from their original container one they were purchased in , or from bulk storage such as a drum or tank, it is important that the proper type of portable container be used. Jay Rathod.

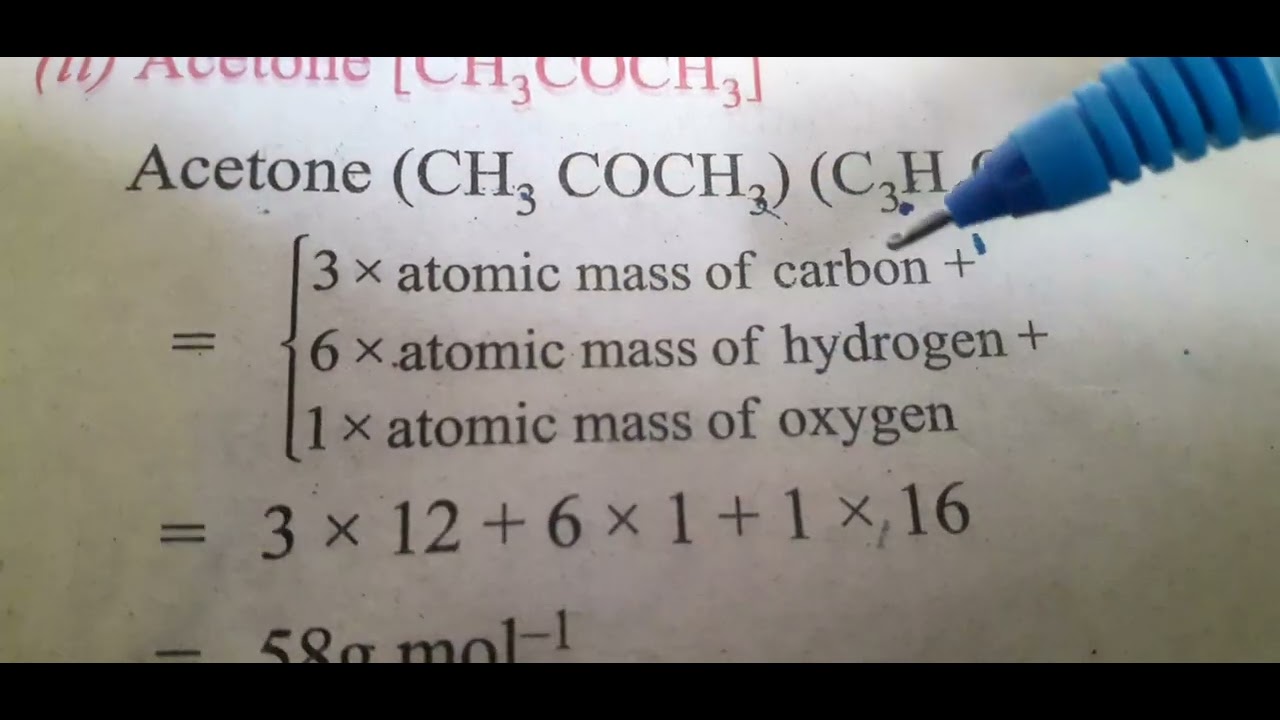
0 thoughts on “C3h6o molar mass”