C2h2 2h2
Zhang, K.
By qwerty April 3, in Homework Help. Any help appreciated. Remember that you want everything to be on the correct side of the arrow when you get to the end, like:. The second reaction reaction by 2 and switch the direction which includes changing the sign for the energy. You need to be a member in order to leave a comment.
C2h2 2h2
It is a hydrocarbon and the simplest alkyne. It is unstable in its pure form and thus is usually handled as a solution. As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. Acetylene was discovered in by Edmund Davy , who identified it as a "new carburet of hydrogen". By heating potassium carbonate with carbon at very high temperatures, he produced a residue of what is now known as potassium carbide , K 2 C 2 , which reacted with water to release the new gas. He also found that acetylene was formed by sparking electricity through mixed cyanogen and hydrogen gases. Berthelot later obtained acetylene directly by passing hydrogen between the poles of a carbon arc. Except in China acetylene production is dominated by partial combustion of natural gas. Since the s, acetylene has mainly been manufactured by the partial combustion of methane. Approximately , tonnes were produced by this method in It is selectively hydrogenated into ethylene , usually using Pd — Ag catalysts. The heaviest alkanes in petroleum and natural gas are cracked into lighter molecules which are dehydrogenated at high temperature:. This last reaction is implemented in the process of anaerobic decomposition of methane by microwave plasma.
Tools Tools. Herring You can adjust your cookie settingsotherwise we'll assume you're okay to continue.
Subsurface chemistry in heterogeneous catalysis plays an important role in tuning catalytic performance. Aiming to unravel the role of subsurface heteroatoms, C 2 H 2 semihydrogenation on a series of Pd catalysts doped with subsurface heteroatom H, B, C, N, P, or S was fully investigated by density functional theory DFT calculations together with microkinetic modeling. The obtained results showed that catalytic performance toward C 2 H 2 semihydrogenation was affected significantly by the type and coverage of subsurface heteroatoms. The Pd-B 0. The essential reason for subsurface heteroatoms in tuning catalytic performance is attributed to the distinctive surface Pd electronic and geometric structures caused by subsurface heteroatoms. In the Pd-B 0. The findings provide theoretically valuable information for designing high-performance metal catalysts in alkyne semihydrogenation through subsurface chemistry.
Now that we understand that chemical reactions occur with a simultaneous change in energy, we can apply the concept more broadly. To start, remember that some chemical reactions are rather difficult to perform. For example, consider the combustion of carbon to make carbon monoxide:. In reality, this is extremely difficult to do. Given the opportunity, carbon will react to make another compound, carbon dioxide:. Is there a way around this? It comes from the understanding that chemical equations can be treated like algebraic equations, with the arrow acting like the equals sign.
C2h2 2h2
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. As acetylene is reactive, unstable, and lighter than the air, the gas is highly flammable and leads to an explosion. Irrespective of being toxic, acetylene is used for welding purposes as it is flammable. To human beings, this compound is no less than an element of risk as to the existence of it in the atmosphere reduces the level of oxygen. Not only it affects human beings but other living species as well disturbing various natural atmospheric cycles for whom oxygen is an integral component. In light of the same, the recommended airborne exposure limit REL of acetylene is set to ppm Ceiling where an amount greater than this can kill human beings by becoming an asphyxiant gas. With this, it becomes crucial to understand the behavioral chemical properties of acetylene to understand why it behaves in such a specific manner. Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic configuration of the participating elements.
Livyatan melvillei vs megalodon
In Peter M. Air Liquide. Tom Mattson Posted April 3, Ponomarev; Sergey M. Acetylene is not especially toxic, but when generated from calcium carbide , it can contain toxic impurities such as traces of phosphine and arsine , which give it a distinct garlic -like smell. Hydrocarbons alkanes alkenes alkynes Cycloalkanes Cycloalkenes Cycloalkynes Annulenes. Almost all of these processes became obsolete with the availability of petroleum-derived ethylene and propylene. Products and Services. Retrieved 30 November Yang, Y. In addition to ethynylation, acetylene reacts with carbon monoxide , acetylene reacts to give acrylic acid , or acrylic esters. Sublimation conditions. Sosa Torres ed. Chemical compound.
.
Li, Y. Beilstein Reference. Accept Cookies Reject Cookies. Hydrocarbons alkanes alkenes alkynes Cycloalkanes Cycloalkenes Cycloalkynes Annulenes. Thermal conductivity. Vinyl acetate is used instead of acetylene for some vinylations, which are more accurately described as transvinylations. Oxyacetylene welding may also be used in areas where electricity is not readily accessible. Archived from the original on 23 February Transform Materials. Copper catalyses the decomposition of acetylene, and as a result acetylene should not be transported in copper pipes. General Chemistry 4th ed. Common applications included coastal lighthouses , [40] street lights , [41] and automobile [42] and mining headlamps.

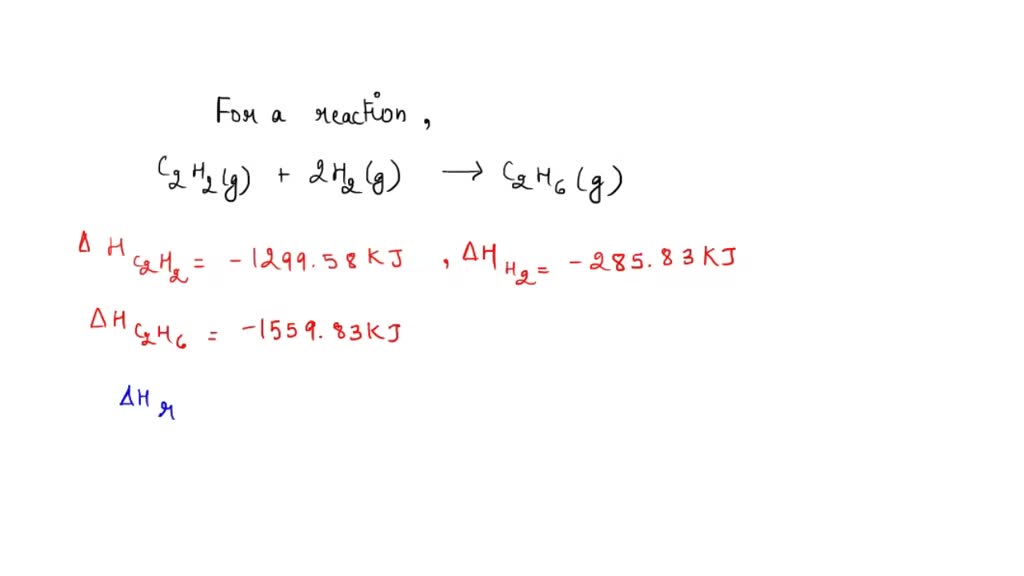
Till what time?
Yes you are talented
Absolutely with you it agree. In it something is also thought excellent.