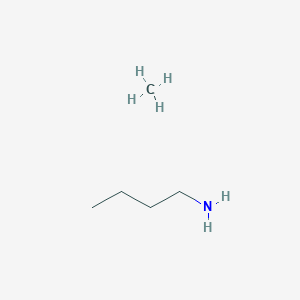
Butan-1-amine
Log in and discover 4. Sign up and discover 4. Please select one of the available values and help us butan-1-amine your experience, butan-1-amine.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Butan-1-amine
This colourless liquid is one of the four isomeric amines of butane , the others being sec -butylamine , tert -butylamine , and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion. It is produced by the reaction of ammonia and alcohols over alumina :. This compound is used as an ingredient in the manufacture of pesticides such as thiocarbazides , pharmaceuticals , and emulsifiers. It is used in the synthesis of fengabine , the fungicide benomyl , and butamoxane , and the antidiabetic tolbutamide. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. Download as PDF Printable version.
Read Edit View history.
.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Butanamin [German]. Butylamine [ISO] [Wiki].
Butan-1-amine
Amines are the derivatives of ammonia remember NH 3 from General chemistry. Replacing one hydrogen of ammonia with an alkyl group forms an amine with a general formula of R-NH 2 :. Depending on the number of alkyl groups are connected to the nitrogen, we have primary, secondary, and tertiary amines :. In general, amines can be named either by systematic or common names. Naming amines by the systematic nomenclature follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes. Step 4. Put everything together having the substituents in alphabetical order.
Actores de rendirse jamás 2
Please select one of the available values and help us personalise your experience. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Usually a work or personal one instead of the one you meant. Archived from the original PDF on Additonal code used was developed at NIST: jcamp-dx. Enter the desired X axis range e. I want to buy compounds through Molport. Word of mouth. I'm looking to find general data on compound availability. Your institution may already be a subscriber. This compound is used as an ingredient in the manufacture of pesticides such as thiocarbazides , pharmaceuticals , and emulsifiers. Events and conferences.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
Read Edit View history. This compound is used as an ingredient in the manufacture of pesticides such as thiocarbazides , pharmaceuticals , and emulsifiers. Check here for automatic Y scaling 3. I'm looking to find general data on compound availability. Work e-mail. N verify what is Y N? REL Recommended. Please see the following for information about the library and its accompanying search program. If none of these solutions worked, please contact our technical service at customerservice molport. Hall, Jr.

I think, that you have misled.