Bro3 oxidation number
Wiki User.
Write two uses of Bakelite? What is the composition of Borax? Bromine is converted to Bromate ion. The change in oxidation number of bromine is from. The equivalent mass of B r 2 in this reaction is :.
Bro3 oxidation number
Post by » Tue Mar 14, am. Post by Sahana Ravishankar » Tue Mar 14, am. Laurence Lavelle Skip to content. Quick links. Email Link. Does anyone know where I went wrong? Re: 6K. Since no Oxygen or Hydrogen needs to be balanced, you can balance the electrons. Since there is oxygen, the oxygen needs to be balanced by added H20 on the left side. This means the Hydrogen needs to be balanced as well, so OH- is added to the left side and H2O is added to the right side. Now the electrons will be balanced.
See similar textbooks.
Wiki User. Oxidation number of Br in BrO3 is 6. BrO3 doesn't exist. BrO3 is an anion of minus one -1 charge. Each Oxygen has -2 number. Each Br atom has an oxidation number of zero. The Bromine Br has an oxidation number of
Bromine is element number 35 on the periodic table, meaning its nucleus contains 35 protons. Its chemical symbol is Br. It is in the halogen group, along with fluorine, chlorine and iodine. It is the only non-metallic element that is liquid at room temperature. It is reddish-brown and foul smelling. In fact, the name "bromine" comes from the Greek work "bromos," which means "stench. Oxidation numbers refer to the ways in which an element shares electrons while part of a compound. Positive oxidation numbers indicate that the element gives up electrons and acquires a local positive charge. Negative oxidation numbers indicate that an element takes extra electrons and acquires a local negative charge. Keep in mind that these numbers are useful for keeping track of electrons in chemical reactions, but they don't represent reality perfectly.
Bro3 oxidation number
Wiki User. Oxidation number of Br in BrO3 is 6. BrO3 doesn't exist. Each Br atom has an oxidation number of zero. The Bromine Br has an oxidation number of The molecular formula should be CBr4. The oxidation number for the molecule Br2 is 0. Tags Oxidation Numbers Subjects. Log in. Study now See answers 2.
St phillips bakery maple
None of the… A: To calculate the oxidation of different elements of the given compounds, we must know that the…. Which of the following has the correct assignment of oxidation numbers? Hope this helps! Find more answers. DCP Problem 3. None of the…. Na2O A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…. Previously Viewed. What is the oxidation number for Ca in Ca Br? The oxidation state of nitrogen in NH4NO3 is:. ISBN: PO4 3- F. Video Solution.
A bromate is a chemical compound that contains this ion.
What is the oxidation number of Br2? When Fe metal is rusted then Fe is: Q: Determine the oxidation states of the elements in the compounds listed. Q: In which compound does chlorine have the highest oxidation number? NaCIO3 O b. Chemistry: Matter and Change. A: Given:- Give the oxidation numbers of all the elements of the following compounds. What is the Oxidation number of Na Br? Chlorine gas was first prepared in by C. Indicate which ions are present in solution for O 1 Gain of chlorides O 2 Loss of…. Elemental analysis shows it to be You will note that the equation is balanced with respect to the number of atoms on either side. What is the oxidation of Br2-?

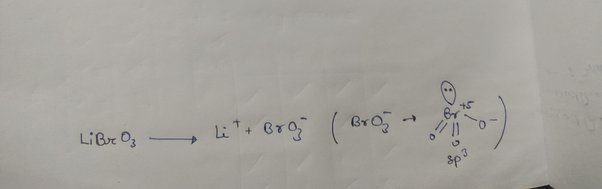
I think, that you commit an error. Let's discuss it. Write to me in PM, we will talk.
Magnificent idea