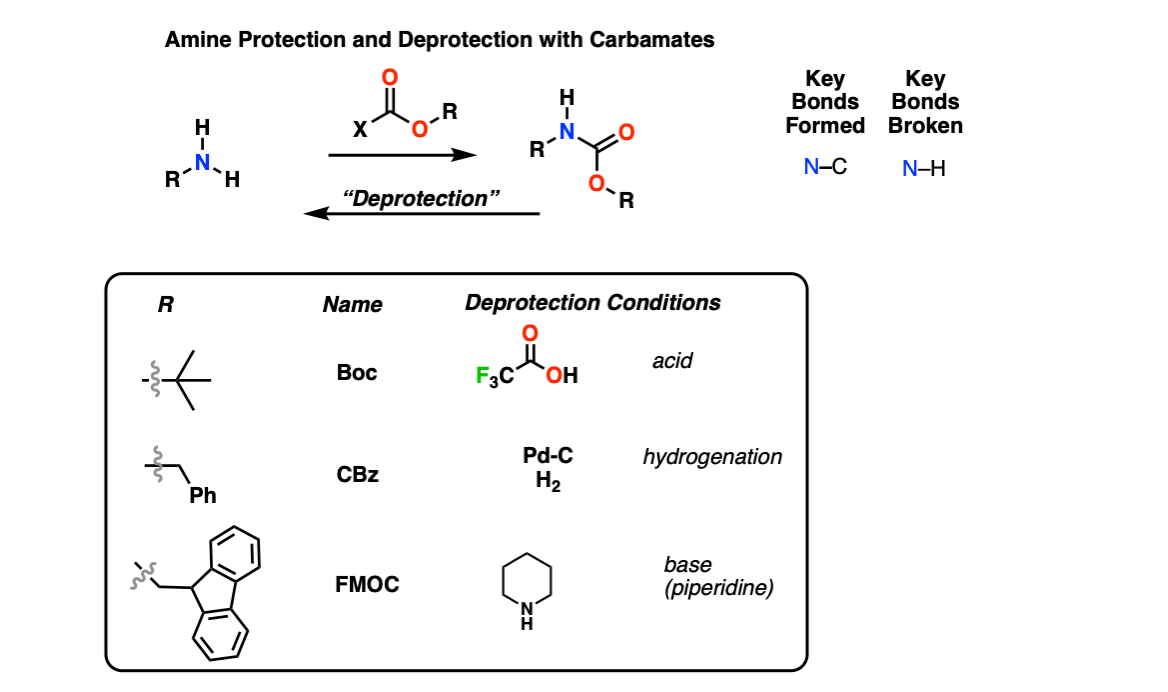
Boc deprotection
The inclusion of an article in this document does not give any indication of safety or operability, boc deprotection. Anyone wishing to use any reaction or reagent must consult and follow their internal chemical safety and boc deprotection procedures and local laws regarding handling chemicals. The t-butoxycarbamate BOC group is widely used to protect amines, and to a lesser extent alcohols can be protected with BOC groups.
The amine attacks a carbonyl site of BOC 2 O, creating a t-butyl carbonate leaving group that breaks down to carbon dioxide gas and t-butoxide. The base then abstracts a proton from the positively charged amine. The protected amine is first protonated by TFA, triggering the production of a t-butyl cation and carbamic acid, which is decarboxylated to yield the amine. Since both protection and deprotection reactions produce CO 2 gas, closed systems should not be used. Jia, X. Environmentally benign N-Boc protection under solvent-and catalyst-free conditions. Synlett , 5,
Boc deprotection
A solution of SM 75 mg, 0. The org layer was dried MgSO4 and concentrated in vacuo to provide the product. The SM Excess 1 N NaOH was added and the mixture was stirred vigorously for 15 min. The org layer was separated, dried MgSO4 , and concentrated in vacuo to provide the product as a brown solid. To a solution of the SM mg, 0. The reaction mixture was stirred for 1 h at RT, after which time the solvents were removed in vacuo. The crude product was used without further purification. To a solution of the SM 1. The reaction mixture was stirred at RT for 2 h, after which time the mixture was co-distilled with DCM times in vacuo. The resulting material was triturated with ether to provide the product as an off-white solid.
Clapham, K.
Federal government websites often end in. The site is secure. We report a mild method for the selective deprotection of the N -Boc group from a structurally diverse set of compounds, encompassing aliphatic, aromatic, and heterocyclic substrates by using oxalyl chloride in methanol. A broader mechanism involving the electrophilic character of oxalyl chloride is postulated for this deprotection strategy. Synthetic organic transformations require the appropriate selection of reagents, catalysts, and most importantly, temporal masking and demasking agents.
Federal government websites often end in. The site is secure. We report a mild method for the selective deprotection of the N -Boc group from a structurally diverse set of compounds, encompassing aliphatic, aromatic, and heterocyclic substrates by using oxalyl chloride in methanol. A broader mechanism involving the electrophilic character of oxalyl chloride is postulated for this deprotection strategy. Synthetic organic transformations require the appropriate selection of reagents, catalysts, and most importantly, temporal masking and demasking agents. The objective for the deployment of relevant masking—demasking agents is to selectively form bonds of interest, whilst minimizing competing reactions with reactive functional groups.
Boc deprotection
N -Boc deprotection deBoc is a common reaction in pharmaceutical research and development, as well as pharma manufacturing. Use of a catalyst lowers the required reaction temperature, and heterogeneous catalysts allow the reaction to be conducted in a continuous flow reactor with a low-boiling solvent, facilitating product separation and enhancing efficiency and productivity relative to a batch process. Boc-protected p -chloroaniline was deprotected with a throughput of 18 mmol p -chloroaniline per h per g cat , sustained over 9 h. Wu, C. Zheng, B. Li, J. Hawkins and S. Scott, React. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
Amul 4 litre ice cream price
After monitoring both reactions for 3 h, the N -Boc l -tryptophan had been completely converted to l -tryptophan in the oxalyl chloride—MeOH system, whereas the HCl—methanol system did not yield an observable deprotection of the N -Boc- l -tryptophan substrate. Naphthylamine entry 2b Prepared according to general deprotection procedure. Zhao Z. Narsimha Reddy A. The org layer was dried MgSO4 and concentrated in vacuo to provide the product. Khaksar S. Debnath S. Taken together, favorable deprotection of aromatics by this deprotection strategy can be attributed to favorable electronic effects of these selected aromatic systems. The amino group is a key functionality that is present in several compounds: natural products, amino acids and peptides. The spectral data of the compound 9a was consistent with the values reported in the literature.
Green, P.
Tamura, Synlett , , 31 , Bergbreiter D. The use of 1,1,1,3,3,3-hexafluoroisopropanol HFIP as solvent and catalyst allows a simple and efficient, chemoselective mono- N -Boc protection of various structurally diverse amines with di- tert -butyl dicarbonate. Chandler G. Merour, G. Bartoli G. Deprotection of structurally diverse N -Boc-amines. It has been shown that the oxalyl chloride mediated reactions with carboxylic acid derivatives can yield isocyanate products. Product: off-white solid Wunderwald S. Torregiani, G. Corresponding author.

As a variant, yes