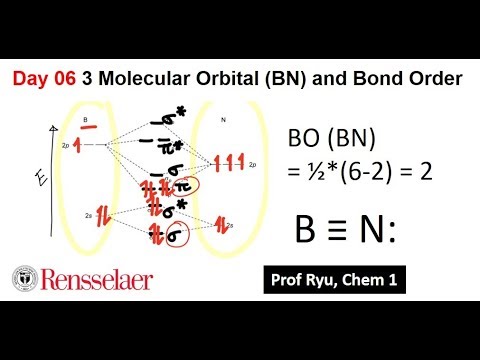
Bn molecular orbital diagram
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electronsand nitrogen has 5 valence electrons, this makes 8 electrons.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Bn molecular orbital diagram
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. In Chapter 2 , we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory A delocalized bonding model in which molecular orbitals are created from the linear combination of atomic orbitals LCAOs , is a delocalized approach to bonding. Molecular orbital theory is a delocalized bonding approach that explains the colors of compounds, their stability, and resonance. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms.
Henderson-Hasselbalch Equation. Gibbs Free Energy. The Quadratic Formula.
.
Valence bond theory is able to explain many aspects of bonding, but not all. To complement this theory, we use another called the molecular orbital MO theory. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. MO theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process called linear combination of atomic orbitals LCAO. The major difference between atomic and molecular orbitals is that atomic orbitals represent electron density in space associated with a particular atom. Molecular orbitals are associated with the entire molecule, meaning the electron density is delocalized spread out over more than one atom. This is the lower energy of the two molecular orbitals and is known as the bonding molecular orbital. Notice in Figure 9. These electrons are stabilized by attractions to both nuclei, and they hold the atoms together with a covalent bond.
Bn molecular orbital diagram
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electrons , and nitrogen has 5 valence electrons, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy. In this case, you need to follow Hund's rule, which states that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. So you end up with 2 unpaired electrons, and paramagnetism of the molecule is explained. How to draw a BN molecular orbital diagram? Dec 11, This is the general MO diagram you need to fill with the valence electrons of BN Boron has 3 valence electrons , and nitrogen has 5 valence electrons, this makes 8 electrons. You have now 2 electrons left, and two orbitals of the same energy.
Pacific fresh food market flyers
Note the Pattern Electrons in nonbonding molecular orbitals have no effect on bond order. Oxides, Peroxides, and Superoxides. Overlap of atomic orbital lobes with the same sign produces a bonding molecular orbital, regardless of whether it corresponds to the sum or the difference of the atomic orbitals. The energies of the molecular orbitals versus those of the parent atomic orbitals can be shown schematically in an energy-level diagram. Intro to General Chemistry 3h 53m. C So the bond order is. Nitric oxide NO is an example of a heteronuclear diatomic molecule. Law of Definite Proportions. Quantum Mechanics 2h 52m. Intro to Acid-Base Titration Curves. In this case, you need to follow Hund's rule, which states that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. Titrations: Weak Acid-Strong Base.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance.
Consequently, electrons in such molecular orbitals are primarily located outside the internuclear region, leading to increased repulsions between the positively charged nuclei. Note the Pattern Molecular orbital theory is a delocalized bonding approach that explains the colors of compounds, their stability, and resonance. Naming Alkanes with Substituents. Writing Formulas of Coordination Compounds. D Calculate the bond order and describe the bonding. If not, would you recommend an oxidation or a reduction to improve stability? The Colligative Properties. Weak Titrate-Strong Titrant Curves. The Electron Configurations: Exceptions. Speed of Light. Molecular Equations. Benzene Reactions. Integrated Rate Law.

0 thoughts on “Bn molecular orbital diagram”