Balzac plus
Write your message below, we will print it on a nice card, placed in your order afterwards.
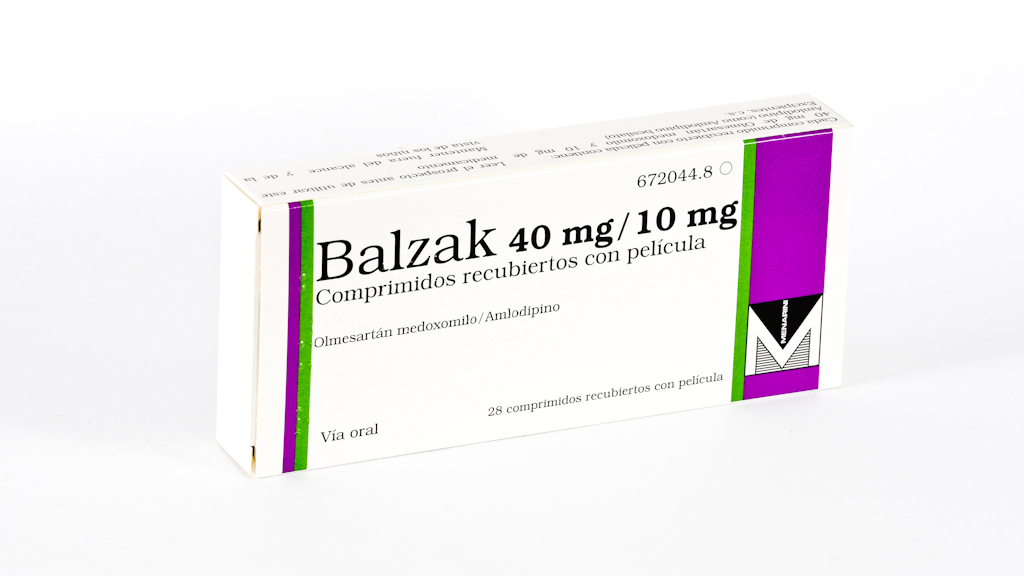
Last updated on Balzak plus is indicated in adult patients whose blood pressure is not adequately controlled on the combination of olmesartan medoxomil and amlodipine taken as dual-component formulation. Balzak plus is indicated as substitution therapy in adult patients whose blood pressure is adequately controlled on the combination of olmesartan medoxomil, amlodipine and hydrochlorothiazide, taken as a dual-component olmesartan medoxomil and amlodipine or olmesartan medoxomil and hydrochlorothiazide and a single-component formulation hydrochlorothiazide or amlodipine. A step-wise titration of the dosage of the individual components is recommended before changing to the triple-component combination. When clinically appropriate, direct change from dual-component combination to the triple-component combination may be considered. Patients controlled on stable doses of olmesartan medoxomil, amlodipine and hydrochlorothiazide taken at the same time as a dual-component olmesartan medoxomil and amlodipine or olmesartan medoxomil and hydrochlorothiazide and a single-component formulation hydrochlorothiazide or amlodipine may be switched to Balzak plus containing the same component doses. Very limited data are available on the use of Balzak plus in patients aged 75 years or older.
Balzac plus
Write your message below, we will print it on a nice card, placed in your order afterwards. Your basket is not compatible with the gift card. Please note, a pre-order product is included in your basket. Combined delivery of your products will take place upon availability. Final promo codes and delivery charges apply at checkout. Subtotal 0. Balzac Paris. New in. The Brand TPR. Second Life. Our shops. Log in. Leopard collection. The Iconics.
When Balzak plus is used in patients with impaired renal function, periodic monitoring of serum concentrations of potassium and creatinine is recommended. The mean volume of balzac plus after intravenous dosing is low 16 - 29 L.
View upcoming auction estimates and receive personalized email alerts for the artists you follow. Filter by media, style, movement, nationality and activity period. Charts on artist trends and performance over time, ready to export. Get your artworks appraised online in 72 hours or less by experienced IFAA accredited professionals. Get the best price for your artwork or collection.
Tratamiento adicional. Insuficiencia renal. Balzak Plus se puede tomar con o sin alimentos. Insuficiencia renal grave ver secciones 4. Segundo y tercer trimestre del embarazo ver secciones 4. Bloqueo dual del sistema renina-angiotensina-aldosterona SRAA :.
Balzac plus
Amlodipine is a Dihydropyridine Calcium antagonist that inhibits the transmembrane influx of Calcium ions into cardiac and vascular smooth muscle. It has greater affinity towards vascular smooth muscle than on cardiac muscle. Amlodipine is peripheral vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and thereby reduces blood pressure.
Staff of life crossword clue
Date de naissance. Free returns in mainland France. We surround ourselves with committed actors suppliers, partners, customers and employees who have the ambition to develop innovative actions having a positive impact on the environment. A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Be careful and be sure to specify the information on the section Name of the medicinal product in the instructions to the drug Balzak plus directly from the package or from the pharmacist at the pharmacy. Renal clearance was approximately 0. The primary endpoint was a composite of fatal CHD or non-fatal myocardial infarction. Our story. Balzak plus is a combination of an angiotensin II receptor antagonist, olmesartan medoxomil, a calcium channel blocker, amlodipine besilate and a thiazide diuretic, hydrochlorothiazide. Total plasma clearance of olmesartan was typically 1. Sweaters and sweatshirts. Unless continued angiotensin II receptor antagonists therapy is considered essential, patients planning pregnancy should be changed to alternative anti-hypertensive treatments which have an established safety profile for use in pregnancy. Acute Myopia and Secondary Angle-Closure Glaucoma Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Balzak plus is not recommended for use in patients aged below 18 years due to a lack of data on safety and efficacy. The address.
HTA esencial. Segundo y tercer trimestre del embarazo. Aldosteronismo primario.
Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy. If diarrhoea does not improve during the week after the discontinuation, further specialist e. No intact olmesartan medoxomil or intact side chain medoxomil moiety have been detected in plasma or excreta. There is no experience of the administration of Balzak plus in patients with a recent kidney transplant or in patients with end-stage renal impairment i. The terminal elimination half-life of hydrochlorothiazide is 10 - 15 hours. Hepatic impairment Balzak plus should be used with caution in patients with mild hepatic impairment. The European Medicines Agency has waived the obligation to submit the results of studies with Balzak plus in all subsets of the paediatric population in essential hypertension. Available in countries. Balzak plus should therefore be administered with caution in these patients. In diabetic patients dosage adjustments of insulin or oral hypoglycaemic agents may be required.

Curious topic
Completely I share your opinion. In it something is also to me it seems it is excellent idea. Completely with you I will agree.