Average atomic mass of sulfur
The average atomic mass of andy spade chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. In your case, average atomic mass of sulfur, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundancewhich is simply the percent abundance divided by How would you find the average atomic mass of sulfur from the following data: S
The natural isotopes of sulfur are listed below: 32 S, Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter.
Average atomic mass of sulfur
Submitted by Jill O. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of Calculate the atomic mass of sulfur symbol S , using data from the table below. Four isotopes of sulfur are sulfur, sulfur, sulfur, and sulfur Abundances for the first three isotopes are What is the average atomic mass of sulfur? Report the answer to five significant figures with units. Calculate the average atomic mass of sulfur if
Login Sign up.
Isotope Atomic mass Da Isotopic abundance amount fraction 32 S Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide. The radioactive isotope 35 S is produced by cosmic-ray interactions with 40 Ar in the atmosphere and decays to 35 Cl with a half-life of 87 days. Sulfur was known as brenne stone for "combustible stone" from which brim-stone is derived.
You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop. The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job.
Average atomic mass of sulfur
Sulfur is the 16 th element of the periodic table. These sulfur facts contain chemical and physical data along with general information and history. Sulfur Periodic Table Cell.
Disney plus log in
Stoichiometric Rate Calculations. Log in. Log in to watch this video Quantum Numbers: Spin Quantum Number. Naming Alcohols. Calculate the average atomic mass of sulfur if Related questions How do you calculate the atomic mass of carbon? Cell Potential: The Nernst Equation. Gibbs Free Energy. What atomic mass does ekasilicon have? Intro to General Chemistry 0.
Atomic mass of all elements along with the rounded off values is mentioned in the chart below.
Subatomic Particles. Pressure Units. Report the answer to five significant figures with units. Maxwell-Boltzmann Distribution. Lewis Dot Symbols. So we have our isotopes here. Log in. Electron Configurations of Transition Metals: Exceptions. Naming Aldehydes. Naming Esters. Born Haber Cycle. First Law of Thermodynamics -. Upgrade to add a comment.

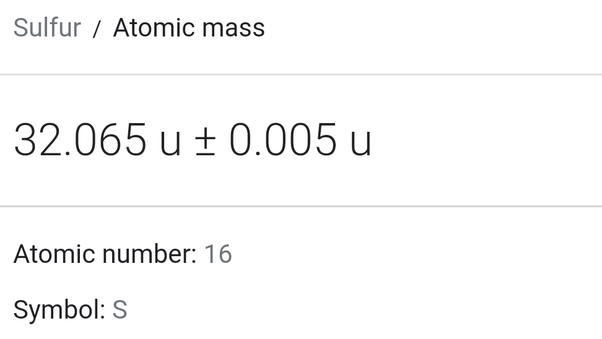
I think, that you are not right. Let's discuss.
Certainly. I agree with told all above. Let's discuss this question. Here or in PM.
Bravo, what phrase..., a brilliant idea