Are ionic bonds stronger than covalent bonds
Atoms separated by a great distance cannot link; rather, they must come close enough for the electrons in their valence shells to interact. But do atoms ever actually touch one another? Most physicists would say no, because the negatively charged electrons in their valence shells repel one another.
Post by Jessica Castellanos » Tue Nov 19, am. Post by jisulee1C » Tue Nov 19, am. Post by joshtully » Mon Oct 26, am. Post by isha dis3d » Wed Oct 28, pm. Post by David Y » Sun Nov 01, am.
Are ionic bonds stronger than covalent bonds
Byju's Answer. Which bond is stronger- ionic or covalent? Open in App. Chemical bonds can be either formed by sharing of electrons or by transfer of electrons, by which the bonding atoms attain an octet[or duplet] state. The bonds formed by sharing of electrons between the bonding atoms are known as covalent bonds. The bonds formed by the transfer of electrons are known as ionic bonds. Generally, ionic bonds are stronger than covalent bonds. Ionic bonds are formed by the transfer of electrons i. While covalent bonds are held together by Van der Waal forces, which are very weak. However, the relative strength of a bond cannot be said accurately as it highly depends on many factors and conditions. For example, in diamond, the carbon atoms are covalently bonded and still it is one of the hardest substances.
Hydrogen, with one electron, will complete its valence shell with two.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy; the stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy see [link]. The stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. The bond energy for a diatomic molecule, D X—Y , is defined as the standard enthalpy change for the endothermic reaction:. Molecules with three or more atoms have two or more bonds. The sum of all bond energies in such a molecule is equal to the standard enthalpy change for the endothermic reaction that breaks all the bonds in the molecule.
Are ionic bonds stronger than covalent bonds
It is essential to remember that energy must be added to break chemical bonds an endothermic process , whereas forming chemical bonds releases energy an exothermic process. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy. The stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy.
Samsung washing machine stops after 1 minute
The opposite charges of cations and anions exert a moderately strong mutual attraction that keeps the atoms in close proximity forming an ionic bond. From what I remember learning, I think that covalent bonds are generally the strongest. Post by joshtully » Mon Oct 26, am Ionic bonds are stronger than covalent bonds. Ions are charged atoms that form when an atom donates or accepts one or more negatively charged electrons. In high school, I was taught that ionic bonds are the strongest, followed by covalent, and finally London forces. Water molecules also strongly attract other types of charged molecules as well as ions. I know in aqueous solutions covalent bonds are usually stronger than ionic and in biology most of the molecules looked at are in aqueous solution. An atom that has an electrical charge—whether positive or negative—is an ion. Standard IX Chemistry. Ions and Ionic Bonds Recall that an atom typically has the same number of positively charged protons and negatively charged electrons. This happens frequently for most atoms in order to have a full valence shell, as described previously. Converting one mole of fluorine atoms into fluoride ions is an exothermic process, so this step gives off energy the electron affinity and is shown as decreasing along the y -axis. Water molecules also repel molecules with nonpolar covalent bonds, like fats, lipids, and oils.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound.
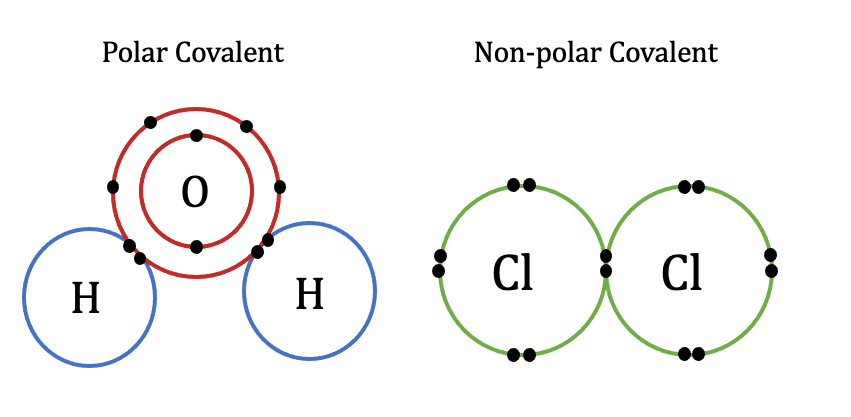
Electron sharing between an atom of carbon and four atoms of hydrogen meets the requirements of all atoms. Methanol, CH 3 OH , may be an excellent alternative fuel. It has a region of weakly positive charge and a region of weakly negative charge. Which are stronger covalent, ionic, and metallic bonds? In this case, the overall change is exothermic. The enthalpy change in this step is the negative of the lattice energy, so it is also an exothermic quantity. This structure is followed by a plus sign, then an oxygen atom with two lone pairs of electrons single bonded to two hydrogen atoms. Molecules with nonpolar covalent bonds are electrically balanced, and have a linear three-dimensional shape. The oxygen atom is single bonded to a hydrogen atom as well. The most familiar example of a polar molecule is water [link]. This happens frequently for most atoms in order to have a full valence shell, as described previously. The bonds are covalent because the electrons are shared: although hydrogen often participates in ionic bonds, carbon does not because it is highly unlikely to donate or accept four electrons. For the ionic solid MX , the lattice energy is the enthalpy change of the process:. Covalent bonds are stronger than ionic bonds. The ionic form of selenium Se , for example, is typically written Se 2—.

0 thoughts on “Are ionic bonds stronger than covalent bonds”