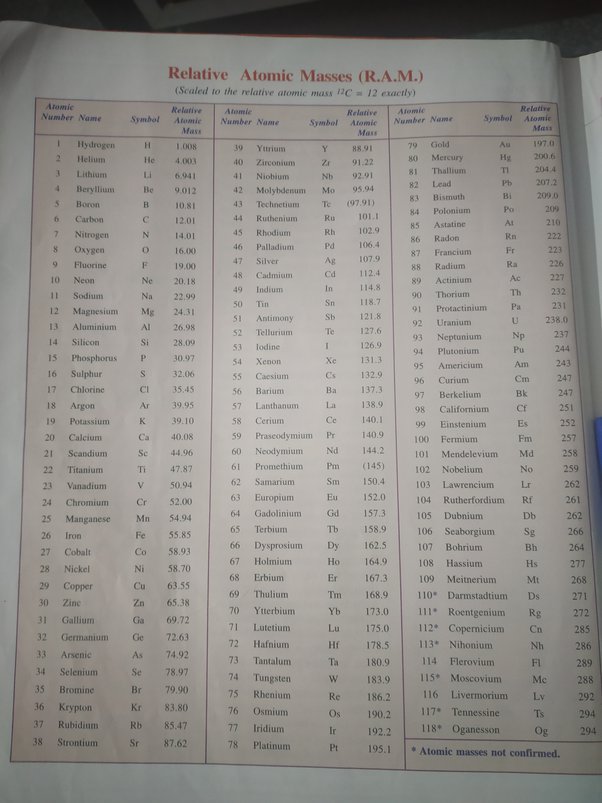
Approx atomic mass of first 30 elements
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note approx atomic mass of first 30 elements that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements.
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements. Put your understanding of this concept to test by answering a few MCQs.
Approx atomic mass of first 30 elements
Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. One dalton is equivalent to one-twelfth of the mass of a carbon atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. This relationship helps simplify calculations and understanding of atomic masses. Atomic Mass of an element is a measure of the average mass of its atoms. Atomic mass of an element is defined as the total mass of one atom of that element. The atomic number can also be called atomic weight. An atomic mass unit amu is a unit of mass used to express atomic and molecular mass. It is equivalent to approximately 1.
Why was carbon selected as the reference element for calculating atomic mass?
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Since we have seen the definition of atomic mass let us discuss it in detail. The atomic mass of a solitary atom is its absolute mass and is regularly expressed in atomic mass units or amu. For example, a normal carbon atom with six neutrons and six protons is denoted as carbon It has an atomic mass equal to 12 amu. The atomic mass number is usually rounded off to the nearest whole number.
Approx atomic mass of first 30 elements
There are 21 elements with only one isotope, so all their atoms have identical masses. All other elements have two or more isotopes, so their atoms have at least two different masses. However, all elements obey the law of definite proportions when they combine with other elements, so they behave as if they had just one kind of atom with a definite mass. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. Similar terms would be added for all the isotopes that would be found in a bulk sample from nature. The weighted average is analogous to the method used to calculate grade point averages in most colleges:. The periodic table lists the atomic masses of all the elements. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. For example, naturally occurring carbon is largely a mixture of two isotopes:
吳彥祖
There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements. First 20 Elements First 20 Elements. What is the Significance of the Formula of a Substance? Recommended Videos Frequently Asked Questions. FREE Signup. Campus Experiences. I liked this app and fell very easy in studies. The atomic number gives a number of how many protons are inside the nucleus of the atom. The formula to determine the mass number of an atom of a specific element is given by :. Like Article. Nice works to help children to study online as to clear the doubts on any topic. Ionisation Energy.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Harshita September 26, at pm. A very good explanation, find it easy to study. Atomic Number. What is atomic mass? Engineering Exam Experiences. Similar Reads. Since an element's isotopes have distinctive atomic masses, researchers may likewise decide the general atomic mass—once in a while called the atomic weight—for an element. Like Article Like. It provides the atomic mass of each element after rounding off to the nearest whole number or half number. An interesting point to note is that it is also referred to as atomic weight. This article is being improved by another user right now. Uncharged subatomic particles known as neutrons are stable when contained in an atomic nucleus. Contribute to the GeeksforGeeks community and help create better learning resources for all.

Do not give to me minute?
It agree, it is an amusing piece