Acetic acid lewis dot structure
Weekly Web Work 7: a. Submissions are no longer accepted. Please type your last name, first name:. Please type the last five digits of your ID number:.
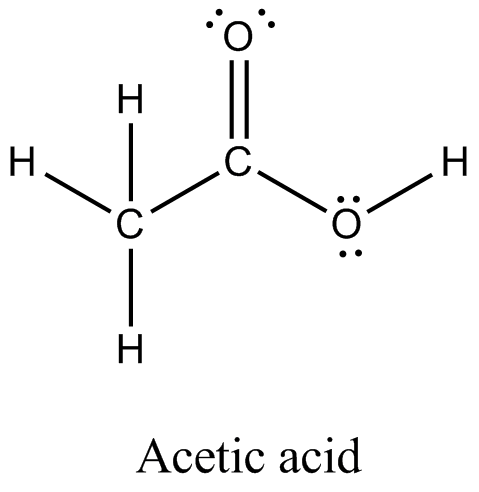
Lewis Dot Structures. This assignment is past due and can no longer be submitted. The purpose of this assignment is to practice drawing Lewis dot structures. You will need to have a pencil and paper to use while working on this assignment. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons.
Acetic acid lewis dot structure
.
Submissions are no longer accepted.
.
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens.
Acetic acid lewis dot structure
We begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction between two identical neutral atoms—for example, the H 2 molecule, which contains a purely covalent bond. Each hydrogen atom in H 2 contains one electron and one proton, with the electron attracted to the proton by electrostatic forces. As the two hydrogen atoms are brought together, additional interactions must be considered Figure 5.
Gardening en español
How is your structure for CS 2 similar to your structure for CO 2? Describe your Lewis dot structure. Let's draw a Lewis structure for chloroform. Exam Info. Please type the last five digits of your ID number:. So, two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons. Weekly Web Work 7: a. How many valence electrons does chloroform have? How many valence electrons does carbon disulfide have? Do you see 24 valence electrons?
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Look at the Lewis structure of acetic acid. Lewis Dot Structures. Exam Info. Why could this be? Remember, 2 electrons are all hydrogen needs to be satisfied. Examine your initial attempt to draw the Lewis structure of chloroform. How is your structure for CS 2 similar to your structure for CO 2? The next step is to write carbon as the central atom. Let's draw a Lewis structure for chloroform. Lecture Audio. Now, place hydrogen and chlorine atoms around the carbon.

0 thoughts on “Acetic acid lewis dot structure”