A hydrogen like atom of atomic number z
The atomic number or nuclear charge number symbol Z of a chemical element is the charge number of an atomic nucleus.
A hydrogen like atom atomic number Z is in a higher excited satte of quantum number n. This excited atom can make a transition to the first excited state by succesively emitting two photon of energies Alternatively, the atom from the same excited state can make a transition to the second excited state by successively emitting twio photon of energy 4. This excited atom can make a transition to first excited state by successively emitting two photons of energies Alternatively, the atom from the same excited state can make a transition to second excited state by successively emitting two phons of energy 4. Determine the values of n and Z. A hydrogen like atom atomic number z is in a higher excited state of quantum number n.
A hydrogen like atom of atomic number z
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 1, Teaches : Physics, Mathematics, English. Teaches : Physics, Biology, Organic Chemistry. Views: 5, Views: 6, Connect with our Physics tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class Hydrogen like atom. A hydrogen like atom of atomic number Z is in an excited state. Solving time: 3 mins.
Recently Updated Pages. Talk to our experts Sign in.
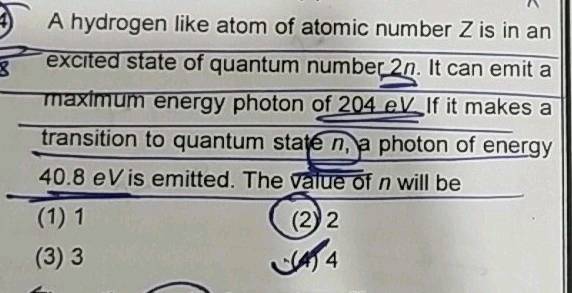
A hydrogen like atom of atomic number Z is in an excited state of quantum number 2 n. It can emit a photon of maximum energy e V. If it makes a transition to quantum state n , a photon of energy The value of n will be. Byju's Answer. Open in App. A hydrogen like atom of atomic number Z is in an excited state of quantum number 2n.
The next system we study is a very useful one, an electron bound to an orbit around a nucleus. The simplest case of such a system is a hydrogen atom which has one electron which orbits its atomic nucleus. In this situation, the electron has both kinetic energy and potential energy described by the electrostatic potential which we will study in detail in Chapter Also, the electron orbits the nucleus in three-dimensional space, so the wave functions, described "standing waves" imposed by the electrostatic potential can no longer be described by simple one dimensional waves with alternative nodes and anti-nodes. Instead, these become much more complex, as depicted by atomic orbitals in the figure below. The details behind these orbitals are outside the scope of this class. However, we can still appreciate what they represent. Like for the one-dimensional case, the orbitals represent the probability of finding the electron around the nucleus and correspond to a specific frequency. The colored regions represent anti-nodes, locations where the probability is highest, while the spaces in between are nodes where probability goes to zero.
A hydrogen like atom of atomic number z
A hydrogen like atom of atomic number Z is in an excited state of quantum number 2 n. It can emit a photon of maximum energy e V. If it makes a transition to quantum state n , a photon of energy The value of n will be. Use app Login. A hydrogen-like atom of atomic number Z is in an excited state of quantum number 2 n.
Hoerbiger parker
Taught by Dolly Panthi. For ordinary nuclei composed of protons and neutrons , this is equal to the proton number n p or the number of protons found in the nucleus of every atom of that element. The next system we study is a very useful one, an electron bound to an orbit around a nucleus. Continue with Google. Teaches : Physics, Biology, Organic Chemistry. The color of the flame is yellow because of these yellow spectral lines. Practice questions on similar concepts asked by Filo students Question 1. Retrieved on A hydrogen atom is in a state of ionization energy 0. Join with a free account. As of , all elements with atomic numbers 1 to have been observed. A hydrogen-like atom is in a higher excited state of quantum number n.
Use app Login. A hydrogen-like atom atomic number Z is in a higher excited state of quantum number n. This excited atom can make a transition to the first state by successively emitting two photons of energies
It can emit a maximum energy photon of eV. Question 3. In a Bohr atom the electron is replaced by a particle of mass time Find important definitions, questions, meanings, examples, exercises and tests below for A hydrogen like atom of atomic number z is in an excited state of quantum number 2n. View All Courses. Submitted by Kevin M. Upto two decimal places Correct answer is between '2. This excited atom can make a transition to the first excited state by successively emitting two proton of energy Add To Playlist Hmmm, doesn't seem like you have any playlists. A hydrogen-like atom atomic number Z is in a higher excited state of quantum number n. Cengage Learning James Stewart. View answers on App. If it makes a transition to quantum state n , a photon of energy

Let's talk on this theme.