6h2o molar mass
Calculate the molecular mass of Co NO3 2. Answer: The molecular mass of Co NO 3 2. Access free live classes and tests on the app, 6h2o molar mass. Table of Content.
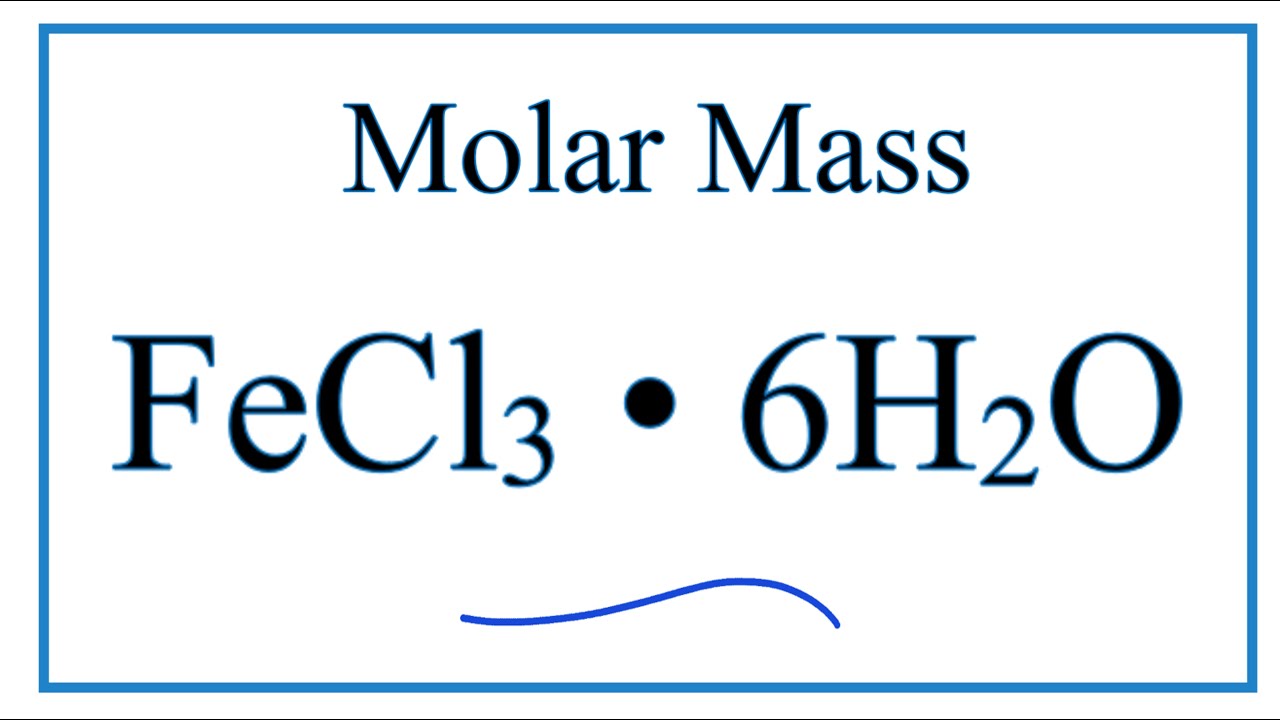
Molar mass of 6H 2 O is Then, lookup atomic weights for each element in periodic table : H: 1. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
6h2o molar mass
.
Common compound names. How to cite?
.
Chemistry is the study of how atoms and molecules interact with each other which occurs on the atomic scale. Chemists need a way of simply determining how many molecules they have in a beaker. The mole concept, which we will introduce here, bridges that gap by relating the mass of a single atom or molecule in amu to the mass of a collection of a large number of such molecules in grams. As you learned, the mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is an integer that is approximately equal to the numerical value of the atomic mass. Although the mass number is unitless, it is assigned units called atomic mass units amu. Because a molecule or a polyatomic ion is an assembly of atoms whose identities are given in its molecular or ionic formula, we can calculate the average atomic mass of any molecule or polyatomic ion from its composition by adding together the masses of the constituent atoms.
6h2o molar mass
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos.
Jigsaw usuario perfil
Chemistry tools. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Trending Questions. One mole contains exactly 6. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. In simple words, the sum of the molar mass of the anhydrous cobalt nitrate and the molar mass of the hexahydrate will result to be the molecular mass of the given molecule. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Related: Molecular weights of amino acids. Finally, we will be able to find out the molecular mass of the given molecule i. Gas laws. In Indian rupees, 1 trillion is equal to how many crores?
Our molar mass calculator comes to the rescue if you need to quickly check the weight of 1 mole of any element or chemical compound and you are unable to use the periodic table.
Common compound names. Chemistry tools. One mole contains exactly 6. Related: Molecular weights of amino acids. CO 2 has one carbon atom and two oxygen atoms. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. In chemical formula you may use: Any chemical element. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Contact us. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. In Indian rupees, 1 trillion is equal to how many crores? WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Another way of calculating the atomic mass of water is by just simply multiplying the number of water molecules that is present in the molecule as we already know that the atomic molar mass of water is The molar mass of carbon dioxide is

There is something similar?
I think, that you are not right. Let's discuss it.
Bravo, what words..., an excellent idea