1 to 30 elements atomic mass
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest.
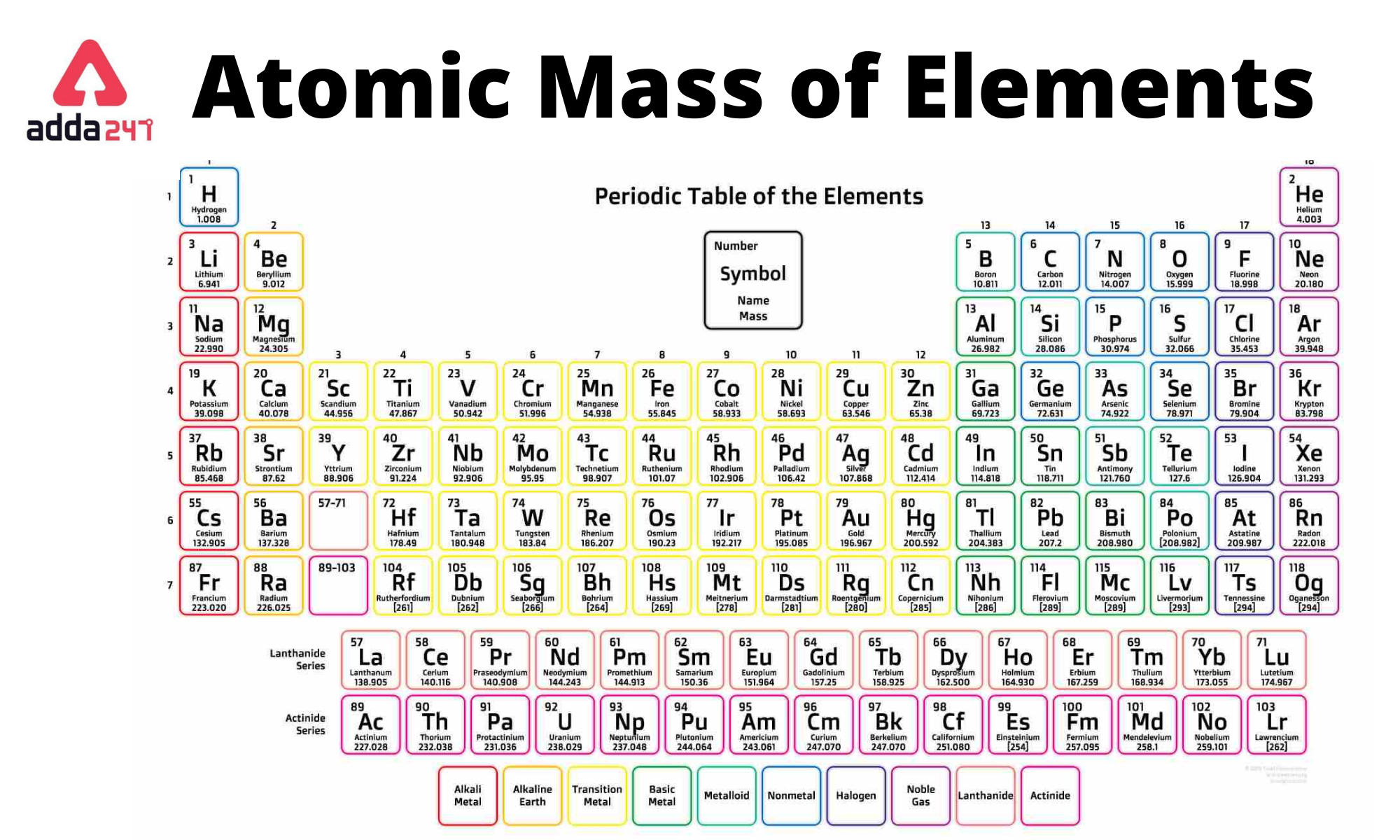
The atomic mass of an element is the average mass of its atoms measured in atomic mass units amu, commonly known as daltons, D. In this article, we will learn about the Atomic Mass of Elements 1 to 30 with Symbols. The absolute mass of a single atom is its atomic mass, which is measured in atomic mass units, or amu. Carbon, for example, is a typical carbon atom with six neutrons and six protons. It has a mass of 12 atomic units.
1 to 30 elements atomic mass
Buka menu navigasi. Tutup saran Cari Cari. Pengaturan Pengguna. Lewati carousel. Karusel Sebelumnya. Karusel Berikutnya. Apa itu Scribd? Akademik Dokumen. Profesional Dokumen. Budaya Dokumen. Pertumbuhan Pribadi Dokumen. Diunggah oleh Prithvi Bhardwaj. Judul dan keterangan yang ditingkatkan AI. Informasi Dokumen klik untuk memperluas informasi dokumen This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements.
The atomic number can also be called atomic weight. Download Adda App.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Since we have seen the definition of atomic mass let us discuss it in detail. The atomic mass of a solitary atom is its absolute mass and is regularly expressed in atomic mass units or amu. For example, a normal carbon atom with six neutrons and six protons is denoted as carbon
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements. Put your understanding of this concept to test by answering a few MCQs. One Mole of a substance is defined as the total number of atoms in 12 grams of Carbon isotope. Atomic mass is the average mass of the protons, neutrons, and electrons in an atom. An Atomic mass unit or one amu is the mass unit equivalent to the one-twelfth mass of one atom of the carbon isotope.
1 to 30 elements atomic mass
Atomic mass of all elements along with the rounded off values is mentioned in the chart below. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro. Jay holds the roles of an author and editor at Periodic Table Guide, leveraging his ability to provide clear explanations on typically unexciting topics related to periodic table. He is passionate to help student, and he finds immense joy in his endeavors to make learning enjoyable and accessible.
Best photoshoot poses for boy
In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. What is the Atomic Mass of Neon? Ionisation Energy. Cations vs Anions What are Ionic Compounds? The atomic mass of sulphur is approximately Here is the table showing the first 30 elements of the periodic table with their atomic masses and atomic number:. Types Of Minerals. The atomic number is simply a digit which is used for placing the elements in the periodic table. This is good app to understand some concepts and this working websites helps to make notes for all the classes. Document Information click to expand document information This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements.
Even though atoms are very tiny pieces of matter, they have mass. Their masses are so small, however, that chemists often use a unit other than grams to express them—the atomic mass unit. Masses of other atoms are expressed with respect to the atomic mass unit.
This was the atomic mass of the first 30 elements. Eurobent Eurostrip - ft1 Eurobent Eurostrip - ft1. The same concept is also used to determine the molar quantities of ionic molecules and compounds. Post My Comment. Start Quiz. Neutrons are uncharged subatomic particles which are stable when bound in an atomic nucleus. Elements are identified based on the number of protons in the nucleus regardless of the number of neutrons present. For example, iron has an atomic mass of Mough aondofa January 17, at pm. What is Plasma and Bose-Einstein Condensate? Atoms and Molecules. Judul dan keterangan yang ditingkatkan AI. Carbon has 6 protons and 6 neutrons, carbon has 6 protons and 7 neutrons, and carbon has 6 protons and 8 neutrons. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Molarity 1-Test Formula

Excuse for that I interfere � here recently. But this theme is very close to me. Write in PM.